More Information
Submitted: March 18, 2025 | Approved: March 24, 2025 | Published: March 25, 2025
How to cite this article: Sharma B, Gupta S, Mukhopadhyay K. Physiological Versus Pathological Cardiac Adaptations in Athletes: Unravelling the Complexities of the Athlete’s Heart through Advanced Echocardiographic Imaging. J Sports Med Ther. 2025; 10(1): 016-024. Available from:
https://dx.doi.org/10.29328/journal.jsmt.1001091
DOI: 10.29328/journal.jsmt.1001091
Copyright License: © 2025 Sharma B, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Athlete’s heart; Cardiac adaptations; Echocardiography and hypertrophic cardiomyopathy
Physiological Versus Pathological Cardiac Adaptations in Athletes: Unravelling the Complexities of the Athlete’s Heart through Advanced Echocardiographic Imaging
Biswajit Sharma1*, Sangeeta Gupta2 and Kishore Mukhopadhyay3
1Research Scholar, Department of Physical Education, Shri Venkateshwara University, NH-24, Venkateshwara Nagar, Near Rajabpur, Gajraula, Distt. Amroha U.P, India
2Ph.D. Supervisor, Shri Venkateshwara University, NH-24, Venkateshwara Nagar, Near Rajabpur, Gajraula, U.P, India
3Associate Professor in Physical Education, Union Christian Training College, Berhampore, Murshidabad, West Bengal, India
*Address for Correspondence: Biswajit Sharma, Research Scholar, Department of Physical Education, Shri Venkateshwara University, NH-24, Venkateshwara Nagar, Near Rajabpur, Gajraula, Distt. Amroha U.P, India, Email: [email protected]
Introduction: The “athlete’s heart” refers to cardiac adaptations from intense training, which can mimic conditions like hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC). This study aims to differentiate physiological and pathological cardiac hypertrophy in athletes using advanced echocardiographic techniques.
Methodology: A literature review of studies from 2000–2024 was conducted using PubMed, Scopus, and Google Scholar, focusing on cardiac remodeling, echocardiographic evaluation, and sudden cardiac death (SCD) risk in athletes.
Discussion: Endurance athletes develop eccentric hypertrophy, while strength athletes exhibit concentric hypertrophy. Physiological adaptations maintain normal function, whereas HCM and ARVC present with structural abnormalities and increased SCD risk. Advanced imaging, including strain and tissue Doppler, is crucial for accurate diagnosis.
Conclusion: Echocardiographic screening is essential for distinguishing normal adaptations from dangerous pathologies in athletes. This study highlights the role of advanced imaging in preventing misdiagnosis and ensuring cardiovascular safety in sports.
Athlete’s heart describes the heart adaptations in structure, function, and electrical patterns caused by consistent athletic training. The athletic heart is beneficial for improving sports performance and health, they may also mimic conditions like hypertrophic cardiomyopathy (HCM) [1].
Understanding physiological changes allows medical professionals to distinguish between normal athletic adaptations and heart diseases in athletes. Echocardiography plays a vital role in assessing athlete’s heart by distinguishing typical exercise-induced cardiac changes from dangerous cardiovascular conditions [2]. Recent scientific studies emphasize exercise intensity and duration as the main elements that drive cardiovascular adaptations. The current interpretation of cardiovascular remodeling offers deeper insights that go past the previous focus on specific sports categories. Although researchers continue to study the effects of age, gender, and ethnicity on cardiac remodeling and try to build a new framework that provides a clearer foundation for therapeutic applications and future studies [3]. According to research by Prior & La Gerche [1] and Palermi, et al. [4], endurance and strength training athletes are mostly demonstrating these cardiac changes. A major meta-analysis by Pluim, et al. [5], confirms that endurance and strength training lead to distinct cardiac adaptations, with endurance athletes having larger left ventricular diameters and strength athletes having thicker walls. Despite these structural differences, both groups maintain normal cardiac function [5]. While strength training leads to thickening of the heart muscle (concentric hypertrophy), endurance training results in a different type of heart muscle growth (eccentric hypertrophy). Activities incorporating both dynamic and static movements like cycling and can produce a combination of these effects [6]. These cardiovascular adaptations frequently include increased cardiac output, thicker walls, and dilation of the left ventricle. The endurance training is commonly associated with these changes, strength athletes also experience similar adaptations, suggesting that both exercise types lead to similar cardiovascular adaptations [7]. Some of these changes, particularly thicker heart walls, can resemble signs of hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC), complicating the differentiation between normal training-induced changes and pathological heart diseases [7-9]. The athlete’s heart may be misdiagnosed as certain cardiac diseases. Conditions like hypertrophic cardiomyopathy (HCM) with its thickened heart muscle and arrhythmogenic right ventricular cardiomyopathy (ARVC), characterized by fibrofatty infiltration can present similarly. While a rare variant exercise-induced ARVC shares traits with both ARVC and the athlete’s heart but without the genetic component found in inherited ARVC. [9,10]. Accurate diagnosis of similar athletic heart conditions requires advanced diagnostic tools like echocardiography to avoid misdiagnosis and ensure appropriate treatment. A deep understanding of the physiological changes in athletes’ hearts is crucial for safe and effective management, enabling optimal performance while minimizing health risks. These advanced imaging techniques are vital for differentiating benign adaptations from potentially serious pathologies needing medical intervention [2,11].
The purpose of the review is to explores how athletes’ hearts adapt both normally and abnormally, highlighting the crucial role of advanced echocardiography in differentiating healthy training effects from potentially serious conditions such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC).
A comprehensive literature search was conducted using Google Scholar, PubMed, Scopus, and Web of Science, focusing on studies related to cardiac adaptations in athletes. Keywords included “athlete’s heart,” “cardiac remodeling,” “echocardiography,” “hypertrophic cardiomyopathy,” and “arrhythmogenic right ventricular cardiomyopathy.” peer-reviewed articles, systematic reviews, and meta-analyses published between 2000 and 2024 were prioritized. Studies were screened based on relevance, methodological quality, and applicability to distinguishing physiological and pathological cardiac adaptations.
Endurance sports and cardiac remodeling: eccentric hypertrophy and functional changes
Running, cycling and swimming among other endurance activities, significantly restructure the heart and particularly enlarging the left ventricle (eccentric hypertrophy). This change is primarily due to the increased blood flow demands of prolonged exercise. Endurance training expands the left ventricle’s capacity (LVEDV) to handle the larger volumes of blood pumped with each heartbeat [6,12,13]. Healthy individuals who engage in endurance training experience significant increases in the size of their left ventricle. These adaptations are most pronounced in men, younger individuals and those already physically fit. Rowing and swimming, as examples of mixed-type exercise and lead to the greatest changes in left ventricular structure [14]. Endurance training induces eccentric hypertrophy and enhances ventricular compliance, improving overall cardiac function by increasing its blood-filling capacity and improving overall heart function [15]. Right ventricular remodeling occurs characterized by enlargement and improved systolic function and correlated with pulmonary artery systolic pressure [7,16]. Endurance athletes’ ability to maintain high performance for extended durations depends critically on their increased capacity for cardiac output [17].
Cardiac remodeling in strength athletes: concentric hypertrophy and functional changes
Weightlifters and other strength athletes develop a unique heart adaptations, which are different from endurance athletes. This typically involves concentric left ventricular hypertrophy—a thickening of the left ventricle’s wall without a significant increase in its internal volume [6,13,18]. Heart remodeling improves the efficiency and pumping strength of the heart muscle , though the increase in blood volume pumped per beat and overall heart output may be less dramatic than in highly trained athletes. While the heart’s main pumping function generally remains normal and sophisticated ultrasound techniques can reveal minor changes in the way the heart muscle deforms [19,20]. The structural changes are adaptations to the high-intensity, short bursts of effort characteristic of strength training [21].
Gender, age, and ethnic variations in athlete’s heart
Cardiac adaptation to exercise varies significantly based on gender age and ethnicity [22]. Men, particularly endurance athletes, typically exhibit greater cardiac changes than women, likely due to hormonal influences on myocardial remodeling. Older athletes may show less pronounced remodeling particularly in left ventricular mass and function, than younger athletes. Furthermore, ethnic differences exist; for example, African-American athletes may demonstrate more pronounced left ventricular hypertrophy than Caucasian athletes and making it challenging to distinguish physiological adaptation from pathology [23]. Di Paolo, et al. [24] found that adolescent African athletes exhibit a higher prevalence of ECG abnormalities, including increased R/S-wave voltage, ST-segment elevation, and T-wave inversion, as well as greater left ventricular wall thickness compared to Caucasian athletes. The study also highlighted country-specific variations in cardiac remodeling, with notable differences between Sub-Saharan and North African athletes [24]. Elite endurance and strength athletes in Asian military males exhibit distinct cardiac adaptations, with greater left ventricular mass index (LVMI) observed in both groups, while lower resting heart rate predicts endurance status, and higher right ventricular systolic pressure characterizes strength athletes [25]. These findings align with Western studies, highlighting universal and ethnicity-specific aspects of athlete’s heart [25].
Asian athletes exhibit unique cardiac adaptations, including smaller left ventricular dimensions and a higher prevalence of anterior T wave inversions on ECG, particularly in Southeast Asians [26,27]. Endurance training induces significant cardiac remodeling, with gender-specific differences in adaptation [28-30]. Compared to other ethnicities, South Asian athletes show fewer ECG anomalies than Afro-Caribbeans and less structural adaptation than Caucasians [27]. Ethnicity-specific cardiovascular screening guidelines are needed to accurately assess Asian athletes’ heart health and SCD risks [31].
Preload and afterload in athlete’s heart
In sports science, preload and afterload are key factors in understanding an athlete’s cardiovascular performance. Preload refers to the myocardial wall stress at end-diastole, which is influenced by factors such as chamber pressure, heart size (radius), and wall thickness [32]. An increased preload enhances stroke volume through the Frank-Starling mechanism, which states that a greater volume of blood filling the heart leads to a stronger contraction and improved endurance capacity [33,34]. Afterload, on the other hand, represents the myocardial wall stress during systolic ejection, which refers to the resistance the heart must overcome to eject blood [32]. A higher afterload increases cardiac workload and oxygen demand, which can influence an athlete’s performance, particularly in high-intensity activities. In endurance athletes, prolonged exposure to increased afterload can lead to left ventricular hypertrophy, an adaptive change that enables the heart to pump more efficiently during exertion [35,36]. Both preload and afterload play a crucial role in the structural and functional adaptations of the athlete’s heart, where the heart undergoes physiological changes to support improved cardiovascular efficiency. Understanding these concepts is essential for optimizing training and monitoring an athlete’s heart health.
Echocardiographic imaging in the athlete’s heart
Endurance athletes’ heart adaptations can be evaluated using echocardiography, which identifies both advantageous and potentially detrimental alterations and including right ventricular dysfunction [37]. Advanced echocardiographic techniques, including tissue Doppler imaging (TDI), strain rate imaging and 3D echocardiography, are crucial for determining the point at which strenuous exercise becomes harmful to the heart. These methods provide more sensitive measurements of left and right ventricular function than standard echocardiography enabling early detection of subtle cardiac remodeling—a key indicator of potential pathology. By assessing cardiac deformation (strain imaging) and evaluating function under stress (exercise echocardiography), researchers can differentiate between healthy training adaptations and pathological conditions like hypertrophic cardiomyopathy (HCM) or arrhythmogenic right ventricular cardiomyopathy (ARVC).Combining these advanced imaging techniques with clinical data is vital for understanding exercise-induced cardiac changes and ensuring the safety of athletes [8,11]. Athlete’s heart involves cardiovascular adaptations that may mimic disease, and echocardiography, along with preparticipation screening, is recommended at key stages (adolescence and age 30–35) to detect conditions like mitral valve prolapse, coronary anomalies, and late-onset cardiac issues [38]. Echocardiographic parameters such as left ventricular (LV) wall thickness, chamber size, and diastolic function are key in distinguishing physiological cardiac remodeling (athlete’s heart) from pathological conditions in athletes [2,8,11]. Recent studies emphasize the use of advanced echocardiographic techniques, including speckle-tracking and 3D imaging, to differentiate between benign adaptations and signs of hypertrophic cardiomyopathy [3,4]. Ali, et al. [39], explore the complexities of distinguishing physiological adaptations in the athlete’s heart from pathological cardiomyopathies using advanced echocardiographic imaging. The study emphasizes the critical role of precise diagnostic criteria and emerging AI technologies in enhancing differentiation, optimizing athlete management, and preventing misdiagnosis-related risks such as sudden cardiac death [39]. Richard, et al. [40] highlight the uncertainty in defining a safe upper limit for peak systolic blood pressure in endurance-trained athletes, suggesting that elevated levels may reflect physiological adaptation rather than pathology. They emphasize the need for athlete-specific guidelines, aligning with the broader challenge of distinguishing physiological versus pathological cardiac adaptations through advanced imaging [40].
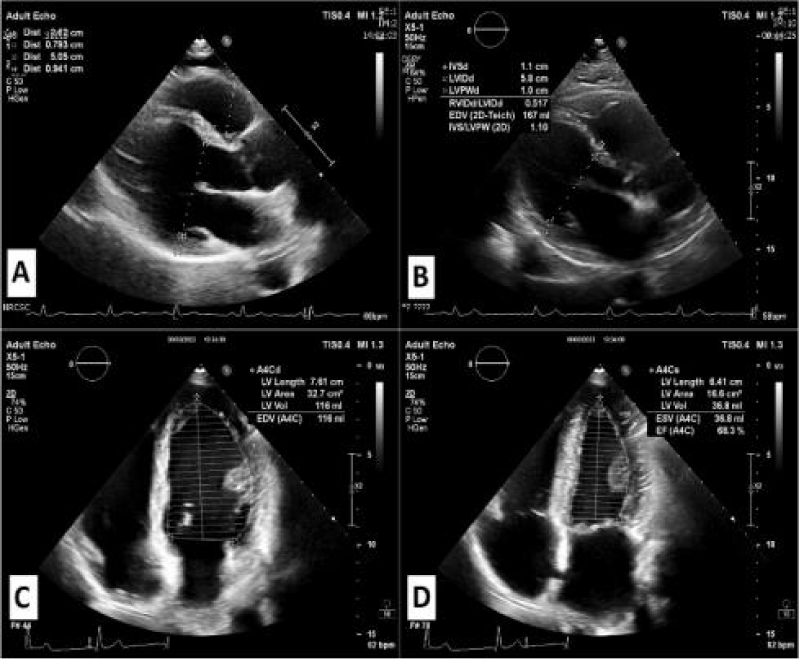
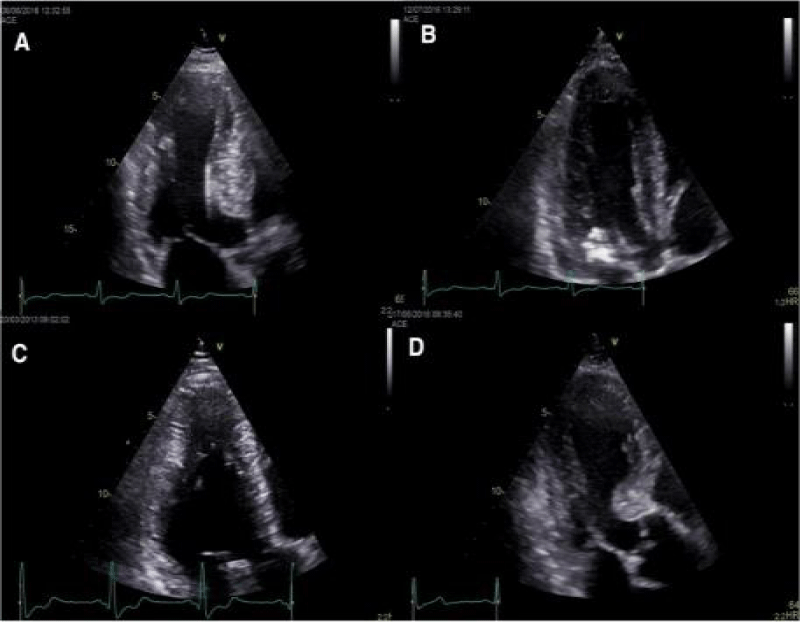
Figures 1,2 represent the echocardiographic images of training induced cardiac hypertrophy [41] and hypertrophic cardiomyopathy [42].
Figure 1: Echocardiographic images of the Athletic Heart.
Figure 2: Echocardiographic images of hypertrophic cardiomyopathy [41,42].
Table 1 Summary of echocardiographic findings in associate with physiological and pathological cardiac changes of sports person.
| Table 1: Summarizing the Echocardiographic Parameters Associated with Physiological (Athlete's Heart) and Pathological Features of Cardiac Remodeling in Athletes: | ||||
| Feature | Physiological Characteristics | Pathological Conditions | Echo Parameters/ Findings | References |
| Left Ventricular (LV) Remodeling | Eccentric hypertrophy in endurance athletes, concentric hypertrophy in strength athletes |
Hypertrophic remodeling, asymmetrical thickening, particularly in the septum and anterior wall |
LV mass and volume increased, preserved function. In HCM, hypertrophy with asymmetric thickening; reduced function may occur | [43,44] |
| Heart Rate (HR) | Lower HR in endurance athletes (bradycardia) | Normal or Increased Heart Rate | ET athletes: lower HR; ST athletes: normal HR; HCM shows normal to elevated HR | [43,45] |
| Left Ventricular Twist (LVT) | Lower LVT in endurance athletes due to more efficient systolic function | Impaired LVT may occur due to myocardial stiffness in HCM | Lower in endurance athletes vs. strength athletes and controls. No change in HCM | [4,43] |
| Diastolic Function (E/A Ratio) |
Enhanced diastolic filling in endurance athletes | Diastolic dysfunction in pathological conditions like HCM | Higher E/A ratio in endurance athletes, lower in strength athletes; HCM may show impaired E/A ratio | [43,46] |
| LV Strain (Speckle Tracking) | Reduced apical circumferential strain in endurance athletes, indicating different cardiac behaviour | Reduced strain in pathological conditions (e.g., HCM) | Apical strain lower in endurance athletes (-21.6%) compared to strength athletes (-26.8%) and controls (-27.8%) | [43] |
| Left Atrium (LA) Remodeling | Mild to moderate enlargement due to increased preload and volume load | Left atrial dilation may occur in conditions like HCM, but functional impairment may be present | Increased LA size in athletes. LA enlargement in HCM may also be observed but associated with atrial arrhythmias | [43,44] |
| Ventricular Wall Thickness | Increased wall thickness, more marked in strength athletes | Increased, often asymmetric, wall thickness in HCM (13-15 mm) | Concentric thickening in strength athletes (normal shape); hypertrophic wall thickening in HCM, often asymmetric | [43,47] |
| Echocardio- graphic Techniques |
Use of conventional and advanced techniques like TDI, STE, and 3D echo for functional analysis | Advanced imaging (MRI, genetic testing) may be used for HCM diagnosis | Standard echocardiography with TDI and 2D strain is used to assess adaptations in the athlete’s heart. MRI and genetic screening in HCM | [10,11] |
| Response to Deconditio-ning | Reversible cardiac changes in athlete’s heart (reduction in LV wall thickness) | No regression in pathological conditions like HCM | Significant LV wall thickness reduction with detraining in athlete’s heart (3mm). No substantial change in HCM | [46,47] |
| Risk of Sudden Cardiac Death (SCD) | Low in athletes with physiological heart remodeling | Higher in athletes with undiagnosed HCM, arrhythmogenic right ventricular cardiomyopathy, or coronary anomalies | Echo helps differentiate between benign adaptation and pathological conditions, with SCD risk evaluation as essential | [11,44,45] |
| Regional LV Strain (Septal Wall) |
Mild reduction in strain, particularly in the septum, due to RV-LV interaction post-exercise. | Significant reduction in septal strain, reflecting hypertrophy and fibrosis in HCM. | LV septal strain decreases post-exercise (e.g., LV septum: -24.4% vs. -21.4%). In HCM, similar or greater reductions due to hypertrophic remodeling. | [48] |
| Preload | Initial stretching of cardiac myocytes before contraction; increases stroke volume via the Frank-Starling mechanism; enhanced in athletes, leading to greater cardiac output. | Excessive preload may contribute to conditions like volume overload, leading to chamber dilation. | Increased end-diastolic volume (EDV); enhanced left ventricular filling; normal or increased chamber size. | [32-34] |
| Afterload | Resistance the heart must overcome to eject blood; chronic adaptation in endurance athletes can lead to left ventricular hypertrophy, increasing stroke volume. | Excessive afterload can contribute to pathological hypertrophy, heart failure, or hypertension- induced cardiac stress. |
Increased left ventricular wall thickness; normal or increased ejection fraction; potential concentric remodeling. |
[32,35,36] |
| HCM: Hypertrophic Cardiomyopathy; ST: Strength Training; ET: Endurance Training | ||||
Summary of key findings
Athlete’s heart shows characteristic echocardiographic adaptations, such as increased LV mass and volume with preserved or enhanced systolic and diastolic function, while pathological conditions like HCM exhibit asymmetric hypertrophy and impaired function [43,44]. Endurance athletes tend to have lower heart rates and higher E/A ratios, while strength athletes show concentric LV remodeling (Pelliccia, et al. 2010; Alasti, et al. 2012). Differentiating athlete’s heart from HCM involves assessing LV wall thickness, diastolic function, and responses to deconditioning [11,43]. The risk of sudden cardiac death is low in athletes with physiological adaptations, but conditions like HCM may increase this risk [11,44]. The role of preload and afterload in shaping the athlete’s heart through physiological adaptations such as increased stroke volume and left ventricular hypertrophy [32-34]. While enhanced preload improves cardiac output via the Frank-Starling mechanism, excessive preload may lead to chamber dilation [32]. Similarly, chronic exposure to increased afterload results in beneficial hypertrophy in athletes but may contribute to pathological conditions like hypertension-induced cardiac stress if excessive [35,36]. Echocardiographic findings support these adaptations, demonstrating increased end-diastolic volume and left ventricular wall thickness in trained individuals [32].
Diastolic function and its implications for athletic performance
Diastolic function significantly impacts overall heart health and athletic capability. Endurance athletes exhibit enhanced early left ventricular (LV) filling, as indicated by higher E/A ratios and improved diastolic velocities, signifying cardiovascular adaptations resulting from sustained exercise. These individuals generally demonstrate effective myocardial relaxation and increased ventricular compliance, which facilitates superior cardiac filling during physical activity . Conversely, strength athletes, who experience concentric hypertrophy, may show changes in diastolic parameters, such as decreased LV compliance or impaired filling, attributable to their thicker myocardial walls [17,49]. Assessing diastolic function using Doppler imaging and strain rate echocardiography offers important insights into how these adaptations affect athletic performance and recovery [50].
Pathophysiological considerations: When the athlete’s heart becomes pathological
Elite athletes often experience left ventricular hypertrophy as a normal physiological adaptation to intense exercise, which can resemble hypertrophic cardiomyopathy (HCM). Accurate differentiation between the two requires a thorough evaluation, including medical history, ECG, echocardiography, and advanced imaging techniques like tissue doppler and MRI [51]. In contrast, arrhythmogenic right ventricular cardiomyopathy (ARVC) is most commonly linked to prolonged, intense physical activity, particularly endurance sports such as marathon running, triathlons, and cycling, due to the sustained strain on the heart, especially the right ventricle. High-intensity interval training (HIIT) and competitive sports like football, rugby, and rowing can also elevate the risk of arrhythmias and contribute to cardiac remodeling. While exercise alone does not directly cause ARVC, it may accelerate disease progression or reveal the condition in individuals genetically predisposed to it [52]. Differentiating between athlete’s heart and arrhythmogenic right ventricular cardiomyopathy (ARVC) requires a careful multiparametric evaluation, including echocardiography, ECG, clinical signs, and imaging techniques like CMR, to avoid misdiagnosis and assess sudden cardiac death risk [53].
Cardiac hypertrophy in athletes may fall within a gray zone between physiological adaptation and pathological hypertrophy, requiring careful assessment to rule out maladaptive changes and underlying conditions. Overtraining, doping, and genetic factors can increase the risk of cardiovascular issues and sudden cardiac death in athletes, highlighting the need for thorough investigation [54]. Sudden cardiac death (SCD) in athletes is often caused by arrhythmias triggered by intense exercise, with cardiomyopathies being the leading cause in athletes under 35 [55]. Hypertrophic Cardiomyopathy (HCM), affecting 1 in 500 people, causes arrhythmias and heart failure symptoms [56,57]. Arrhythmogenic Cardiomyopathy (ACM), prevalent in 1:1000 to 1:5000, leads to arrhythmias and sudden cardiac death (SCD) in young athletes [58]. Genetic mutations in beta-myosin heavy chain (MYH7), myosin binding protein C (MYBPC3) (HCM), and desmoplakin proteins like DSP, plakophilin PKP2 arrhythmogenic cardiomyopathy (ACM) are involved [59]. Screening for these conditions remains a topic of debate [60]. A study of 357 athletes who died suddenly revealed that sudden arrhythmic death syndrome (SADS) and myocardial diseases, such as arrhythmogenic right ventricular cardiomyopathy (ARVC) and left ventricular fibrosis, were the leading causes of sudden cardiac death (SCD), with intense exertion triggering 61% of cases [61]. The findings emphasize the importance of early detection and tailored preventive strategies to address these risks [61]. While the majority of athletes exhibit standard physiological changes in their hearts, some individuals may develop pathological issues that resemble these adaptations. Hypertrophic cardiomyopathy (HCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), and various other types of cardiomyopathies can show structural alterations that overlap with characteristics of the athlete’s heart. Important diagnostic indicators of pathological conditions include abnormal myocardial workload, decreased myocardial efficiency, and altered strain patterns, which can be detected using advanced echocardiographic methods. These conditions necessitate thorough evaluation to ensure precise diagnosis and suitable management [11,21,62]. Intense exercise can cause heart remodeling, potentially leading to reduced ventricular function, arrhythmias, and increased risk of heart disease, requiring differentiation from pathological conditions and consideration of performance-enhancing drug effects [63]. Echocardiography plays a crucial role in screening athletes for structural heart diseases that may lead to sudden cardiac death (SCD), distinguishing between physiological adaptations and early-stage cardiomyopathies using advanced techniques like strain imaging [64]. Its high sensitivity and specificity make it essential for primary prevention, helping identify at-risk athletes before clinical symptoms arise [64]. Exercise stress echocardiography (ESE) helps differentiate between normal athletic heart adaptations and pathologies, offering insights into cardiac function, exercise capacity, and arrhythmias. Despite challenges like interpretation variability, its growing use in sports cardiology is crucial for preventing sudden cardiac events in athletes [4]. Table 2
| Table 2: Pathophysiological Considerations in the Athlete's Heart. | ||
| Topic | Details | References |
| Physiological Adaptation | Left ventricular hypertrophy (LVH) occurs as a normal response to intense exercise, resembling hypertrophic cardiomyopathy (HCM). | Khamis & Mayet [51] |
| Pathological Hypertrophy | Requires differentiation from HCM and other heart conditions, involving comprehensive assessment with ECG, echocardiography, and advanced imaging. | Khamis & Mayet [51] |
| Exercise-Induced ARVC | Prolonged, strenuous physical activity, particularly endurance sports like marathon running, triathlons, and cycling, can induce exercise-related ARVC due to sustained strain on the heart, particularly the right ventricle. | Gasperetti, et al. [52] |
| Differentiation Between Athlete’s Heart and ARVC | Diagnosing ARVC versus athlete's heart requires careful multiparametric evaluation, including echocardiography, ECG, clinical signs, and imaging techniques like CMR, to avoid misdiagnosis and assess sudden cardiac death risk. | D’Ascenzi, et al. 2018 [53] |
| Risk Factors for SCD | Overtraining, doping, and genetic factors increase risk of sudden cardiac death (SCD) due to arrhythmias during exercise. | Abibillaev & Kocyigit [54]; Maron, et al. [55]; Finocchiaro, et al. [61] |
| Causes of SCD in Athletes | Cardiomyopathies (HCM, ACM, ARVC) are the leading causes of SCD in athletes under 35, with arrhythmias as a common trigger during intense exertion. | Maron, et al. [56]; Alcalai, et al. [57]; Finocchiaro, et al. [61]; Corrado, et al. [58] |
| Genetic Factors | Mutations in genes such as MYH7 (HCM), MYBPC3 (HCM), and desmoplakin (ACM) are linked to arrhythmias and SCD in young athletes. | El Hadi, et al. [59] |
| Screening Debate | Ongoing debate over the necessity and effectiveness of genetic screening for heart conditions like HCM and ACM in athletes. | Modesti, et al. [60] |
| Key Diagnostic Indicators | Abnormal myocardial workload, reduced efficiency, and altered strain patterns detected via advanced echocardiographic techniques. | Jumadilova, et al. [21]; D’Andrea, et al. [11]; La Gerche, et al. [62] |
| Role of Echocardiography | Echocardiography, including strain imaging, plays a crucial role in distinguishing between physiological and pathological cardiac changes in athletes. | Radmilovic, et al. [64] |
| Exercise Stress Echocardiography (ESE) | ESE helps differentiate normal athletic heart adaptations from pathological changes, assessing exercise capacity and arrhythmia risk. | Palermi, et al. [10] |
| SCD Risk and Exercise | Intense exertion, especially with underlying heart conditions (e.g., ARVC, left ventricular fibrosis), can trigger arrhythmic SCD in athletes. | Finocchiaro, et al. [61]; Carbone, et al. [63] |
Represent the findings of the research report of different researcher regarding pathophysiological considerations of cardiac hypertrophy.
The “athlete’s heart” is a normal physiological adaptation to intense exercise but can closely resemble conditions such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic cardiomyopathy (ACM). As a result, a thorough evaluation, including imaging and genetic screening, is essential for accurate diagnosis [51,59,64]. Additionally, exercise-induced arrhythmogenic right ventricular cardiomyopathy (ARVC), commonly associated with endurance sports like marathons, triathlons, and cycling, can also mimic ARVC due to the sustained strain on the heart, particularly the right ventricle [52]. Differentiating between athlete’s heart and ARVC can be challenging and requires a comprehensive, multiparametric evaluation, including imaging techniques such as cardiac magnetic resonance (CMR), to avoid misdiagnosis and assess the risk of sudden cardiac death (SCD) [53]. Early detection and accurate differentiation are crucial in preventing SCD in athletes [56,60,61].
Exercise-induced right heart remodeling in athletes
Long-term exercise and high pulmonary artery pressures frequently cause substantial remodeling of the right heart in endurance athletes. Increased right atrial (RA) and right ventricular (RV) dimensions are part of this remodeling, and they are necessary to sustain efficient cardiac output during endurance exercises. Furthermore, because endurance athletes have higher cardiac output, they may also have higher pulmonary artery systolic pressures (PASP). However, when strength athletes exercise in various ways, their right heart exhibits more subtle modifications [16,65]. Clinicians who work with athletes must have a thorough understanding of the particular alterations in the right heart and how they affect performance [20]. A meta-analysis by Dawkins, et al. [66]
demonstrated that athletes exhibit elevated right ventricular (RV) systolic pressure, increased tricuspid annular displacement and velocity, and region-specific functional adaptations, distinguishing physiological remodeling from pathology. These findings underscore the importance of assessing both RV structure and function in evaluating the athlete’s heart [66].
The Table 3 distinguishes physiological cardiac adaptations, which maintain normal function and reverse with deconditioning, from pathological hypertrophy, which involves persistent structural changes and increased SCD risk [6,8]. ECG differences, such as increased QRS voltage in athletes versus T-wave inversions in pathology, highlight the importance of advanced imaging for accurate diagnosis [22,24,38].
| Table 3: Physiological vs. Pathological Cardiac Adaptations in Athletes. | ||
| Feature | Physiological Athlete’s Heart | Pathological (HCM & ARVC) |
| LV Structure | Eccentric/concentric cardiac hypertrophy, normal function [6]. |
Marked hypertrophy, asymmetric thickening [8]. |
| Diastolic Function | Normal or enhanced [6]. | Impaired relaxation, high filling pressures [11]. |
| ECG Findings | Increased QRS voltage, bradycardia [22]. |
T-wave inversions, ST-segment depression [24]. |
| Response to Rest | Reversible with deconditioning [37]. |
Persistent hypertrophy [8]. |
| SCD Risk | Low [38]. | High [8]. |
The phenomenon of the “athlete’s heart” represents a fascinating interplay between the cardiovascular system and prolonged intense physical training. While many of the structural and functional changes observed in athletes are benign and supportive of enhanced performance, it is essential to distinguish between physiological adaptations and pathological conditions, particularly in the context of elite athletic populations. Advances in echocardiography, including techniques like tissue Doppler imaging, strain rate imaging, and 3D echocardiography, play a critical role in identifying these adaptations and detecting early signs of cardiovascular disease. These advanced imaging modalities enable a more precise understanding of the athlete’s heart and help clinicians differentiate between normal physiological remodeling and pathological conditions, such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC), which present with overlapping features.
Athletes undergo distinct cardiac adaptations based on their training type —endurance athletes tend to exhibit eccentric hypertrophy, while strength athletes demonstrate concentric hypertrophy. Factors such as age, gender, and ethnicity also modulate these adaptations, requiring tailored diagnostic and management approaches. Moreover, while the majority of athletes demonstrate healthy cardiac remodeling, there is a critical need for vigilance in detecting pathological conditions, especially given the potential risks of sudden cardiac death (SCD) and arrhythmias. Early detection through comprehensive screening, including echocardiographic assessment at key life stages, is pivotal in ensuring the long-term health and safety of athletes.
Continued research into the long-term cardiovascular effects of intense physical activity, including the potential risks of arrhythmias, atrial enlargement, and deconditioning, is necessary. It is also crucial to explore the implications of genetic predispositions and the effects of performance-enhancing drugs on cardiac health. Ultimately, understanding the athlete’s heart is essential for not only optimizing performance but also preventing the tragic outcomes associated with undiagnosed cardiovascular conditions.
- Prior DL, La Gerche A. The athlete’s heart. Heart. 2012;98(12):947–955. Available from: https://doi.org/10.1136/heartjnl-2011-301329
- Albaeni A, Davis JW, Ahmad M. Echocardiographic evaluation of the athlete’s heart. Echocardiography. 2021;38(6):1002–1016. Available from: https://doi.org/10.1111/echo.15066
- Beaudry R, Haykowsky MJ, Baggish A, La Gerche A. A modern definition of the athlete’s heart—for research and the clinic. Cardiol Clin. 2016;34(4):507–514. Available from: https://doi.org/10.1016/j.ccl.2016.06.001
- Palermi S, Cavarretta E, D’Ascenzi F, Castelletti S, Ricci F, Vecchiato M, et al. Athlete’s heart: A cardiovascular step-by-step multimodality approach. Rev Cardiovasc Med. 2023;24(5):151. Available from: https://doi.org/10.31083/j.rcm2405151
- Pluim BM, et al. The athlete’s heart: a meta-analysis of cardiac structure and function. Circulation. 2000;101(3):336–344. Available from: https://doi.org/10.1161/01.CIR.101.3.336
- Fagard R. Athlete’s heart. Heart. 2003;89(12):1455–1461. Available from: https://doi.org/10.1136/heart.89.12.1455
- Baggish AL, Yared K, Wang F, Weiner RB, Hutter AM, Picard MH, et al. The impact of endurance exercise training on left ventricular systolic mechanics. AJP Heart Circ Physiol. 2008;295(3):H1109–H1116. Available from: https://doi.org/10.1152/ajpheart.00395.2008
- Flanagan H, Cooper R, George KP, Augustine DX, Malhotra A, Paton MF, et al. The athlete’s heart: insights from echocardiography. Echo Res Pract. 2023;10(1). Available from: https://doi.org/10.1186/s44156-023-00027-8
- Darden D, Scheinman MM, Hoffmayer KS. Exercise-induced arrhythmogenic right ventricular cardiomyopathy: Reverse remodeling with detraining. HeartRhythm Case Rep. 2022;8(9):599–603. Available from: https://doi.org/10.1016/j.hrcr.2022.06.003
- Palermi S, Sperlongano S, Mandoli GE, Pastore MC, Lisi M, Benfari G, et al. Exercise stress echocardiography in athletes: Applications, methodology, and challenges. J Clin Med. 2023;12(24):7678. Available from: https://doi.org/10.3390/jcm12247678
- D’Andrea A, Bossone E, Radmilovic J, Caso P, Calabrò R, Russo MG, et al. The role of new echocardiographic techniques in athlete’s heart. F1000Res. 2015;4:289. Available from: https://doi.org/10.12688/f1000research.6745.1
- Rafailakis L, Deli CK, Fatouros IG, Tsiokanos A, Draganidis D, Poulios A, et al. Functional and morphological adaptations in the heart of children aged 12–14 years following two different endurance training protocols. Sports. 2023;11(8):157. Available from: https://doi.org/10.3390/sports11080157
- Pittaras A, Faselis C, Doumas M, Grassos C, Kokkinos P. Physical activity and cardiac morphologic adaptations. Rev Cardiovasc Med. 2023;24(5):142. Available from: https://doi.org/10.31083/j.rcm2405142
- Morrison BN, George K, Kreiter E, Dixon D, Rebello L, Massarotto RJ, et al. Effects of endurance exercise training on left ventricular structure in healthy adults: A systematic review and meta-analysis. Eur J Prev Cardiol. 2023;30(9):772–793. Available from: https://doi.org/10.1093/eurjpc/zwad023
- Hedge ET, Brazile TL, Hughson RL, Levine BD. Plasticity of the heart in response to changes in physical activity. J Physiol. 2024. Available from: https://doi.org/10.1113/jp284158D’
- D'Andrea A, Riegler L, Golia E, Cocchia R, Scarafile R, Salerno G, et al. Range of right heart measurements in top-level athletes: The training impact. Int J Cardiol. 2011;164(1):48–57. Available from: https://doi.org/10.1016/j.ijcard.2011.06.058
- Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2014;28(2):236–244. Available from: https://doi.org/10.1016/j.echo.2014.09.013
- Malakhova SM, Syvolap VV, Potapenko MS. Features of cardiac remodeling depending on the mode of training session. Zaporozhye Med J. 2020;(5). Available from: https://doi.org/10.14739/2310-1210.2020.5.214735
- Li P, Zhang Y, Li L, Chen Y, Li Z, Liu S, Hua S. Assessment of left ventricular systolic function by non-invasive pressure-strain loop area in young male strength athletes. Cardiovasc Ultrasound. 2020;18(1). Available from: https://doi.org/10.1186/s12947-020-00227-w
- D'Andrea A, Carbone A, Radmilovic J, Russo V, Fabiani D, Di Maio M, et al. Myocardial work efficiency in physiologic left ventricular hypertrophy of power athletes. J Cardiovasc Echography. 2022;32(3):154–159. Available from: https://doi.org/10.4103/jcecho.jcecho_11_22
- Jumadilova D, Rakhmanov Y, Khissaatdinov N, Zhankorazova A, Toktarbay B, Khamitova Z, et al. Differences in cardiac mechanics assessed by left ventricular hemodynamic forces in athletes and patients with hypertension. Sci Rep. 2024;14(1). Available from: https://doi.org/10.1038/s41598-024-78560-7
- Papadakis M, Wilson MG, Ghani S, Kervio G, Carre F, Sharma S. Impact of ethnicity upon cardiovascular adaptation in competitive athletes: relevance to preparticipation screening. Br J Sports Med. 2012;46(Suppl 1):i22–i28. Available from: https://doi.org/10.1136/bjsports-2012-091127
- Grewal J. Left ventricular function and exercise capacity. JAMA. 2009;301(3):286. Available from: https://doi.org/10.1001/jama.2008.1022
- Di Paolo FM, Schmied C, Zerguini YA, Junge A, Quattrini F, Culasso F, et al. The athlete’s heart in adolescent Africans. J Am Coll Cardiol. 2012;59(11):1029–1036. Available from: https://doi.org/10.1016/j.jacc.2011.12.008
- Liu P, Tsai K, Lima JA, Lavie CJ, Lin G. Athlete’s heart in Asian Military males: The CHIEF Heart Study. Front Cardiovasc Med. 2021;8. Available from: https://doi.org/10.3389/fcvm.2021.725852
- Yeo TJ, Wang M, Grignani R, McKinney J, Koh LP, Tan FHY, et al. Electrocardiographic and echocardiographic insights from a prospective registry of Asian elite athletes. Front Cardiovasc Med. 2022;8. Available from: https://doi.org/10.3389/fcvm.2021.799129
- Ghani S, Al-Khafaji Z, Reed M, Zaidi A, Sheikh N, Narain R, et al. 157 Cardiovascular Adaptation In Athletes Of South Asian Origin: Relevance To Universal Implementation Of Pre-Participation Cardiovascular Screening. Heart. 2013;99(Suppl 2):A92.1–A92. Available from: https://doi.org/10.1136/heartjnl-2013-304019.157
- Tan V, Koh XH, Tan F, Hazli H, Ling LH, Yeo TJ. The impact of elite endurance activity on cardiac remodelling in Asians: an echocardiographic case control study. Eur Heart J. 2022;43(Supplement_2). Available from: https://doi.org/10.1093/eurheartj/ehac544.2487
- Lin G, Liu P, Lima JAC, Lavie CJ. Abstract P067: Elite Female Athlete’s Heart of Asians: The Chief Heart Study. Circulation. 2022;145(Suppl_1). Available from: https://doi.org/10.1161/circ.145.suppl_1.p067
- Liu M, Liu P, Tsai K, Lima JAC, Lavie CJ, Lin G. Asian Female Athlete’s Heart: The CHIEF Heart Study. PubMed. 2023;39(6):888–900. Available from: https://doi.org/10.6515/acs.202311_39(6).20230306f
- Finocchiaro G, Radaelli D, D'Errico S, Bhatia R, Papadakis M, Behr ER, et al. Ethnicity and sudden cardiac death in athletes: insights from a large United Kingdom registry. Eur J Prev Cardiol. 2024;31(12):1518–1525. Available from: https://doi.org/10.1093/eurjpc/zwae146
- Norton JM. Toward Consistent Definitions For Preload And Afterload. AJP Adv Physiol Educ. 2001;25(1):53–61. Available from: https://doi.org/10.1152/advances.2001.25.1.53
- Little RC. Cardiac preload, afterload, and heart failure. Arch Intern Med. 1982;142(4):819. Available from: https://doi.org/10.1001/archinte.1982.00340170179027
- S M. [Interdependence of preload, afterload and contractility: their relation to cardiac function curve]. PubMed. 1992;41(11):1782–1787. Available from: https://pubmed.ncbi.nlm.nih.gov/1460755
- Cahill NS, O'Brien M, Rodahl A, Allen JF, Knight D, Dolphin C. A pilot study on left ventricular dimensions and wall stress before and after submaximal exercise. Br J Sports Med. 1979;13(3):122–129. Available from: https://doi.org/10.1136/bjsm.13.3.122
- Toischer K, Rokita AG, Unsöld B, Zhu W, Kararigas G, Sossalla S, et al. Differential cardiac remodeling in preload versus afterload. Circulation. 2010;122(10):993–1003. Available from: https://doi.org/10.1161/circulationaha.110.943431
- King G, Wood MJ. The heart of the endurance athlete assessed by echocardiography and its modalities: "Embracing the delicate balance." Springer Science+Business Media New York; 2013. Available from: https://doi.org/10.1007/s00246-013-0681-7
- Niederseer D, Rossi VA, Kissel C, Scherr J, Caselli S, Tanner FC, et al. Role of echocardiography in screening and evaluation of athletes. Heart. 2020;107(4):270–276. Available from: https://doi.org/10.1136/heartjnl-2020-317996
- Ali NB, Esfahani SA, Scalia IG, Farina JM, Pereyra M, Barry T, et al. The role of cardiovascular imaging in the diagnosis of athlete’s heart: Navigating the shades of Grey. J Imaging. 2024;10(9):230. Available from: https://doi.org/10.3390/jimaging10090230
- Richard NA, Hodges L, Koehle MS. Elevated peak systolic blood pressure in endurance‐trained athletes: Physiology or pathology? Scand J Med Sci Sports. 2020;31(5):956–966. Available from: https://doi.org/10.1111/sms.13914
- Zholshybek N, Khamitova Z, Toktarbay B, Jumadilova D, Khissamutdinov N, Dautov T, et al. Cardiac imaging in athlete’s heart: current status and future prospects. Cardiovasc Ultrasound. 2023;21:21. Available from: https://doi.org/10.1186/s12947-023-00319-3
- Mandeş L, Roşca M, Ciupercă D, Popescu BA. The role of echocardiography for diagnosis and prognostic stratification in hypertrophic cardiomyopathy. J Echocardiogr. 2020;18:137–148. Available from: https://doi.org/10.1007/s12574-020-00467-9
- Santoro A, Alvino F, Antonelli G, Caputo M, Padeletti M, Lisi M, et al. Endurance and Strength Athlete’s heart: Analysis of myocardial deformation by speckle tracking Echocardiography. J Cardiovasc Ultrasound. 2014;22(4):196. Available from: https://doi.org/10.4250/jcu.2014.22.4.196
- Barbier J, Ville N, Kervio G, Walther G, Carré F. Sports-Specific Features of Athlete’s Heart and their Relation to Echocardiographic Parameters. Herz. 2006;31(6):531–543. Available from: https://doi.org/10.1007/s00059-006-2862-2
- Alasti M, Omidvar B, Jadbabaei MH. Heart and athlete. J Tehran Heart Center. 2010;5(1):1–8.
- Atchley AE, Douglas PS. Left ventricular hypertrophy in athletes: morphologic features and clinical correlates. Cardiol Clin. 2007;25(3):371–382. Available from: https://doi.org/10.1016/j.ccl.2007.06.009
- Pelliccia A. Athlete’s heart and hypertrophic cardiomyopathy. Curr Cardiol Rep. 2000;2(2):166–171. Available from: https://doi.org/10.1007/s11886-000-0015-4
- Stewart GM, Chan J, Yamada A, Kavanagh JJ, Haseler LJ, Shiino K, et al. Impact of high-intensity endurance exercise on regional left and right ventricular myocardial mechanics. Eur Heart J Cardiovasc Imaging. 2016;jew128. Available from: https://doi.org/10.1093/ehjci/jew128
- Abdelmetaal FK, Saleh HA, Abdelaziz OA, Souliman SM, Samy BM. Effect of training on diastolic function of the heart in male volleyball players. Egypt Rheumatol Rehabil. 2013;40(1):50–55. Available from: https://doi.org/10.7123/01.err.0000427585.89200.49
- Dalen H, Letnes JM, Hoydal MA, Wisløff U. Diastolic function and dysfunction in athletes. Eur Heart J Cardiovasc Imaging. 2024. Available from: https://doi.org/10.1093/ehjci/jeae15
- Khamis RY, Mayet J. Echocardiographic assessment of left ventricular hypertrophy in elite athletes. Heart. 2008;94(10):1254–1260. Available from: https://doi.org/10.1136/hrt.2008.153783
- Gasperetti A, James CA, Cerrone M, Delmar M, Calkins H, Duru F. Arrhythmogenic right ventricular cardiomyopathy and sports activity: from molecular pathways in diseased hearts to new insights into the athletic heart mimicry. Eur Heart J. 2020;42(13):1231–1243. Available from: https://doi.org/10.1093/eurheartj/ehaa821
- D’Ascenzi F, Solari M, Corrado D, Zorzi A, Mondillo S. Diagnostic differentiation between arrhythmogenic cardiomyopathy and athlete’s heart by using imaging. JACC Cardiovasc Imaging. 2018;11(9):1327–1339. Available from: https://doi.org/10.1016/j.jcmg.2018.04.031
- Abibillaev D, Kocyigit F. Athletic heart adaptation, pathological hypertrophy and sudden cardiac death. Heart Vessels Transplant. 2020;4(2):55. Available from: https://doi.org/10.24969/hvt.2020.199
- Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes: clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199–204.
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92(4):785–789. Available from: https://doi.org/10.1161/01.cir.92.4.785
- Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. Available from: https://doi.org/10.1111/j.1540-8167.2007.00965.x
- Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. Available from: https://doi.org/10.1093/eurheartj/ehz669
- El Hadi H, Freund A, Desch S, Thiele H, Majunke N. Hypertrophic, dilated, and arrhythmogenic cardiomyopathy: where are we? Biomedicines. 2023;11(2):524. Available from: https://doi.org/10.3390/biomedicines11020524
- Modesti PA, Casolo G, Olivotto I, Pellegrino A. Sudden death in young athletes: is it preventable? Eur J Intern Med. 2022;104:13–20. Available from: https://doi.org/10.1016/j.ejim.2022.06.00
- Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. JACC Heart Fail. 2016;67(18):2108–2115. Available from: https://doi.org/10.1016/j.jacc.2016.02.062
- La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, MacIsaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2011;33(8):998–1006. Available from: https://doi.org/10.1093/eurheartj/ehr397
- Carbone A, D’Andrea A, Riegler L, Scarafile R, Pezzullo E, Martone F, et al. Cardiac damage in athlete’s heart: when the “supernormal” heart fails! World J Cardiol. 2017;9(6):470. Available from: https://doi.org/10.4330/wjc.v9.i6.470
- Radmilovic J, D'Andrea A, D'Amato A, Tagliamonte E, Sperlongano S, Riegler L, et al. Echocardiography in athletes in primary prevention of sudden death. J Cardiovasc Echogr. 2019;29(4):139–148. Available from: https://doi.org/10.4103/jcecho.jcecho_26_19
- Okoshi K, Okoshi MP. Doppler echocardiography in athletes from different sports. Med Sci Monit. 2016;22:1234–1241. Available from: https://doi.org/10.12659/MSM.883829
- Dawkins TG, Curry BA, Wright SP, Meah VL, Yousef Z, Eves ND, et al. Right ventricular function and region-specific adaptation in athletes engaged in high-dynamic sports: a meta-analysis. Circ Cardiovasc Imaging. 2021;14(5). Available from: https://doi.org/10.1161/circimaging.120.012315
- Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, et al. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J Am Coll Cardiol. 2014;64(12):1257–1266. Available from: https://doi.org/10.1016/j.jacc.2014.03.062
- Jouffroy R, Benaceur O, Toussaint J, Antero J. Echocardiographic assessment of left ventricular function 10 years after the ultra-endurance running event Eco-Trail de Paris® 2011. Int J Environ Res Public Health. 2022;19(14):8268. Available from: https://doi.org/10.3390/ijerph19148268
- Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol. 2005;45(8):1364–1367. Available from: https://doi.org/10.1016/j.jacc.2005.02.015
- Morganroth J. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82(4):521. Available from: https://doi.org/10.7326/0003-4819-82-4-521
- Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, et al. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol. 2011;589(22):5443–5452. Available from: https://doi.org/10.1113/jphysiol.2011.217125