Review Article
Role of physical activity in cancer survival and recurrence: A narrative review from relationship evidence to crucial research perspectives

J Maury1, P Senesse1 and G Ninot1,2*
1Montpellier Cancer Institute, Montpellier, France
2EA4556 Research Unit, University of Montpellier, Montpellier, France
*Address for Correspondence: Gregory Ninot, Montpellier Cancer Institute, 208 Avenue des Apothicaires, 34298 Montpellier, France, Tel: (+33) 4 67 14 54 99; Email: gregory.ninot@umontpellier.fr
Dates: Submitted: 30 November 2018; Approved: 11 December 2018; Published: 12 December 2018
How to cite this article: Maury J, Senesse P, Ninot G. Role of physical activity in cancer survival and recurrence: A narrative review from relationship evidence to crucial research perspectives. J Sports Med Ther. 2018; 3: 102-117. DOI: 10.29328/journal.jsmt.1001034
Copyright License: © 2018 Maury J, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cancer; Physical activity; Exercise; Survival; Recurrence; Evidence
Abstract
Purpose: The benefits of Physical Activity (PA) considered as a major supportive care in cancer patients, on survival, and recurrence risk is largely disseminated in public communication. However, these data must be taken with caution. The main objectives were to review the evidence and limits of studies reported regarding the post-diagnosis PA role on cancer survival and recurrence risk to secondly discuss of research perspectives on PA programs.
Method: The narrative review included all published or ongoing studies in English during the last 20 years related to PA, survival and recurrence risk with a systematic search on main databases.
Results and discussion: The current evidences regarding the PA role on survival and recurrence risk were only based on cohort studies, mainly in breast cancer. The major methodological limits identified as the lack of PA change assessment, PA level assessed largely by self-reported methods and the significant inter- but also intra- variability make the interpretation of data very. Beyond the use of rigorous RCT, the major issue is to develop adapted and personalized interventions to progressively increase PA level overtime in cancer survivors.
Conclusion: Despite the lack of causal relationship between post-diagnosis PA, survival and recurrence risk, the review underlines several interesting research perspectives. The future PA interventions, using innovative tools and integrated to the “real-life” will argued for the potential antitumoral PA role growing in literature.
Introduction
The prevalence of cancer has been steadily increasing for many years, making it the second leading global cause of death [1]. This increase is due to the optimization of diagnostic tools, main and adjuvant treatments, as well as recent developments in personalized medicine, thus promoting longer survival with cancer [2,3]. These advances justified the inclusion of supportive care during treatment and recurrence prevention periods. Physical activity (PA) is now considered as a component supportive care strategy. The role of PA interventions was to improve quality of life, more recently to decrease psychological concerns such as fatigue, anxiety, depression, pain, self-esteem trouble, body image concerns and social isolation [4]. Many cohort studies and randomized clinical trials have respectively indicated and showed benefits of PA interventions on these markers predominantly in breast cancer, but also in prostate, colon or blood cancer [5-7].
Over time, PA has also been recognized to improve specific physiological parameters. Several meta-analyses have shown strong experimental evidence to conclude that PA improve cardiorespiratory parameters [7-9], body composition and muscle mass [7,8,10], inflammatory markers, glucose metabolism or immune system [11-13]. Given the benefits observed in the literature on these bio-psycho-social parameters, all international expert panels recommend implementation of adapted physical activity (APA) programs, as an important non-pharmacological intervention in addition to the main treatments during all care phases for most cancers and even immediately after diagnosis [14-16]. An APA program may alleviate adverse events related to cancer and its treatment, and may improve cancer treatment efficacy [17]. In fact, emerging evidence from pre-clinical studies indicate that exercise training may control cancer progression through direct effects on tumour intrinsic factors (growth rate, metastasis, tumour metabolism, and immunogenicity of the tumour) and regulates tumour growth through interplay with systemic factors [17].
Several meta-analyses and epidemiological studies seem to support this assumption [14,18,19]. After the cancer diagnosis, physical inactivity is raised in cancer patients due to the effect of main treatments on functional capacities that could be increased by psychological components such as anxiety, depression and reduced self-esteem [20]. Insidiously, this physical inactivity contributes not only to physiological deconditioning, psychological and social consequences of cancer described above but also to long term deleterious effects associated with increased mortality and recurrence risk, thus constituting a major public health issue. In this sense, different meta-analyses seem to report a positive association between the practice of PA after diagnosis, improvement of survival and decreased risk of recurrence [15,21-25]. These data contribute to the emerging idea that PA may be considered as a specific adjuvant therapy in cancer with a potential antitumor role [17]. Even if the benefits of PA on survival and the risk of recurrence after cancer treatment is largely disseminated and admitted in public communication, some authors highlighted numerous methodological limitations and large variability of proofs between the different cancer type suggesting that these data must be taken with caution [14,16,23,26]. Now regarding all these parameters, the issue well recognized in the scientific community is to conduct randomized controlled trials with rigorous methodologies to optimize the current recommendations and to define APA programs that can be integrated into the “real life” care of cancer patients.
First, the narrative review presents the evidence and the limits of published studies regarding the post diagnosis PA role on cancer survival and recurrence. Secondly, the review details research perspectives to optimize the development and evaluations of APA programs.
Literature Search Method
Data sources and searches
For this narrative review, studies published in English in the last 20 years (between January 1998 and May 2018) were identified by a systematic search on Pubmed, ScienceDirect, Web of Science and Motrial. Combinations of the following terms were used (MeSH Terms): cancer OR physical activity OR exercise OR survival OR recurrence OR tumor OR free-disease survival. The research algorithms were combined with filters to specially identify randomized controlled trials (RCT) and cohort studies. In addition, publications integrated to the present narrative review were checked from relevant meta-analysis published in each cancer type. Regarding cancer survival and recurrence parameters, we also searched the RCT in progress in ClinicalTrials.gov (U.S. National Library of Medicine), INCa database, Chinese Clinical Trial Registry (ChiCTR), Cochrane Central Register of Controlled Trials, European Clinical Trials Register, International Clinical Trials Registry Platform (ICTRP), ISRCTN registry, and UMIN Clinical Trials Registry (UMIN-CTR).
Data collection process
After screening consequently titles, abstracts and full texts, two researchers (JM, PhD and GN, PhD) independently reviewed and selected eligible publications aiming to investigate the benefits of PA on reporting outcomes (survival, recurrence). Studies were selected if they included adult patients with a cancer diagnosis established before the study beginning. We focused our searches on the main cancers studied in the literature: breast, lung, colon, prostate, pancreatic and hemopathy. Studies were excluded if they did not provide any details on methodology and/or PA program. Extracted data included the first author’s name, year of publication, tumor stage and site, treatment status, study objective, sample size, study design and PA program methodology (intensity, frequency, modalities and duration), adherence rate and delivery mode (supervision, group, individual etc.). The results on survival, free-survival and recurrence parameters were extracted. When there was insufficient information, the relevant corresponding author was contacted.
Synthesis of data
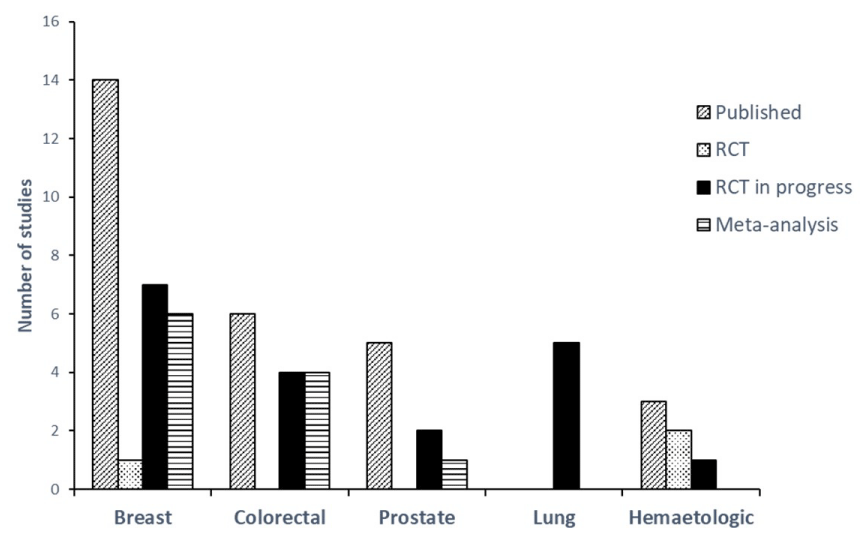
In order to illustrate the discussion of the present narrative review, a global description of studies was firstly established to identify the current evidence available about PA effects on survival, free-survival and recurrence. For this, the number of published, in progress studies (only RCT) and meta-analysis were summarized in a graph (Figure 1). In addition, tables were established to detail the main information of published studies (Table 1) or in progress RCT (Table 2)
| Table 1. Characteristics of the 28 studies identified about the relation between post diagnosis physical activity, survival and recurrence in cancer patient. | |||||
| Author, year, country | Cancer type, stage and phase | Study design, subjects, sex | PA measurement and characteristics | Follow-up duration | Main results |
| Beasley and et al. 2012 (USA) [45] | Breast cancer Stages I – III Post treatment (18 to 48 months after) |
Cohort (Life after Cancer Epidemiology study) 13302 women |
Semi-quantitative questionnaires (Median of 23 months post-diagnosis) No initial PA evaluation |
Until date of death or recurrence orlast contact | Recreational PA level ≥10 MET-hours/week associated with a 27% reduction in all-cause of mortality and 25% in breast cancer mortality. No association with breast cancer recurrence. |
| Holick and et al. 2008 (USA) [46] | Breast cancer All cancer stages 2 years after diagnosis |
Cohort (Collaborative Women’s Longevity Study) 4482 women |
CWLS questionnaire Time spend to recreational PA during the previous week Assessment 2 years after diagnosis No initial PA evaluation |
Median follow-up : 6 years | Women who engaged in ≥2.8 MET-h/wk of recreational PA had a 35% to 49% decreased risk of death from breast cancer and all-cause of mortality. No information on cancer recurrence. |
| Holmes and et al. 2005 (USA) [31] | Breast cancer Stage I-III (30 à 55 years old) |
Cohort (Nurses’ Health study) 2987 women |
Leisure-time PA (questionnaire) evaluated at least 2 years post-diagnosis | Median follow-up : 8 years | Women who engaged in ≥3 MET-h/wk of (dose-response effect) leisure-time PA had a decreased risk of death from breast cancer, all-cause of mortality and recurrence in particular in women with hormone responsive tumors. |
| Nechuta and et al. 2016 [82] | Breast cancer Stages I-III 2 years after diagnosis |
Cohort (After Breast Cancer Pooling Project) 6596 women estrogen receptor-positive |
Self-reported information on recreational PA measured on average 2,1 years after diagnosis No initial PA evaluation |
Median follow-up time for mortality: 12 years Mediand follow-up time for recurrence: 10,6 |
Higher level of recreational PA (≥17,4 MET-hours/week) is inversely associated with late all-cause of mortality and 25% in breast cancer mortality. No association with breast cancer recurrence. |
| Irwin and et al. 2011 [79] | Breast cancer Stages I-III |
Cohort (Women’s Health Initiative) 4463 women |
Self-reported information on recreational PA measured on average 1,8 years after diagnosis. No initial PA evaluation |
Median follow-up time for mortality: 3,3 years | Moderate to vigourous level of recreational PA (≥9 MET-hours/week) is inversely associated with a 46% reduction of all-cause of mortality and 25% in breast cancer mortality. No data on breast cancer recurrence. |
| De Glas and et al. 2014 [76] | Breast cancer All cancer stages 1 year after diagnosis |
Cohort (The Tamoxifen Exemestane Adjuvant Multicenter) 435 women |
PA questionnaire at 1 and 2 years post diagnosis. | 2 years | Overall survival is statically significantly better for patients who were relatively active. No association with breast cancer-specific survival and recurrence-free periods. |
| Courneya and et al. 2014 (Canada) [27] | Breast cancer Stages I-III During treatment |
Randomized controlled trial (Supervised Trial of Aerobic versus Resistance Training) 242 women |
AP program aerobic vs resistance training) during chemotherapy (3 sessions/week) Aerobic training : 15 to 45 minutes on cycloergometer (70% VO2peak) Resistance training : 9 exercises (8-12 repetitions) |
8 years (Median follow-up: 89 months) | No significant association between PA, disease-free survival, overall survival and recurrence-free interval. |
| Friedenreich and et al. 2009 [77] | Breast cancer All cancer stages |
Cohort (Alberta Cancer Registry) 1231 women |
Lifetime PA Questionnaire at baseline (recreational, occupational and household activity). No PA change evaluation |
Minimal follow-up: 8,3 years for recurrence and 10,3 years for deaths | Highest recreational PA activity is associated with a decrease in breast cancer death and all deaths. Moderate intensity recreational activity decreased the risk of recurrence |
| Bradshaw and. 2014 [73] | Breast cancer (Long Island Breast Cancer Study Project) All cancer stages |
Cohort 1423 women |
Recreational PA was assessed through structured interviews at baseline and follow-up using a semi-questionnaire | Median survival time among women in our study: 12,7 years | Survival was improved among women who were highly active after diagnosis (9.0 MET h/week) compared to inactive women (0 MET h/week) for all-cause and breast cancer-specific mortality. No data on breast cancer recurrence. |
| Williams, 2014 [86] | Breast cancer All cancer stages |
Cohort (National Runners’ and Walkers’ Health Surveys) 986 women |
PA questionnaire in order to identify runner’s vs walkers at baseline | Median follow-up : 9 years | For the 986 runners and walkers combined, breast cancer mortality decreased an average of 23.9% by MET-H/day. There was a significantly greater decrease in death risk for running than walking. No data on breast cancer recurrence |
| Bertram and et al. 2010 [42] | Breast cancer Stages I-III Post treatment |
Cohort (Women’s Healthy Eating and Living) 2361 women |
PA questionnaire at baseline and at various follow-up points | Median follow-up : 7,1 years | Moderate to vigorous PA level at baseline (≥3 MET-hours/week) is associated with a 53% reduction of mortality risk. Adherence to PA guidelines (10 MET-h/week) was associated with a 35% lower mortality risk. No association of PA with breast cancer recurrence |
| Chen and et al. 2011 (China) [75] | Breast cancer Stages I-III 12 months following diagnosis |
Cohort (Shanghai Breast Cancer Survival Study) 4826 women |
PA level was assessed (by interviews) at 6, 18 and 36 months postdiagnosis | Median follow-up : 4,3 years | PA during the first 36 months postdiagnosis was inversely associated with total mortality and disease-specific mortality |
| Sternfeld and et al. 2009 [84] | Breast cancer Stages I-III |
Cohort (Life After Cancer Epidemiology) 1970 women |
Self-reported frequency and duration of work-related, household and recreational activities during the 6 months prior the inclusion | Median follow-up : 7,3 years | A statically significant protective association between PA and all-cause mortality. No significant association between PA, risk of recurrence and breast cancer mortality Only a trend for higher levels (≥3 MET-h/week) of PA versus lowest level was reported on these parameters. |
| Hellmann and et al. 2010 [78] | Breast cancer All cancer stages |
Cohort (Copenhagen City Heart Study) 528 women |
A self-administered questionnaire and clinical examination by trained personnel were used to PA assessment. No initial PA evaluation |
Median follow-up : 7,8 years | A moderate physical activity of 2–4 h/week and a high physical activity of more than 4 h/week showed no association with survival after breast cancer diagnosis. No data on breast cancer recurrence. |
| Meyerhardt and et al. 2009 [81] | Colorectal cancer nonmetastic Stages I-III After diagnosis |
Cohort (Health Professionals Follow- up Study) 668 men |
Leisure-time PA questionnaire within 15 months after diagnosis | Median follow-up : 8,6 years | High PA level (≥27 MET-h/week) was associated with improved colorectal cancer-specific mortality, overall mortality. |
| Baade and et al. 2011 [71] | Colorectal cancer Stages I-III After diagnosis |
Cohort 1089 men 736 women |
PA level assessed 5 months and 12 months after diagnosis | Median follow-up : 5 years | Participants with some level of PA (≥150 minutes/week) following diagnosis had 25% to 28% lower risk of all-cause mortality within 5 years of diagnosis than sedentary participants. Increases in PA from five to 12 months postdiagnosis was associated with reduced colorectal specific mortality by 32% to 36% and 31% for all-cause mortality. |
| Meyerhardt and et al. 2006a [32] | Colorectal cancer Stages I-III After diagnosis |
Cohort (Nurses’ Health study) 573 women |
Leisure-time PA (questionnaire) evaluated at least 1 year but no more than 4 years post-diagnosis | Median follow-up : 9,6 years | High PA level (≥18 MET-h/week) compared with low level of PA (≤3 MET-h/week) was associated with an improve in colorectal cancer-specific mortality, overall mortality. |
| Meyerhardt and et al. 2006b [47] | Colorectal cancer Stage III After diagnosis |
Cohort (Cancer and Leukemia Group B) 832 patients |
PA level assessed by questionnaire 6 months after completion of adjuvant therapy | Median follow-up : 3,8 years | High PA level (≥18 MET-h/week) compared with low level of PA (≤3 MET-h/week) was associated with a reduction in recurrence-free survival and overall survival. |
| Campbell and et al. 2013 [74] | Colorectal cancer Stages I-III After diagnosis |
Cohort (Cancer Prevention Study) 1271 men 991 women |
Self-reported recreational and leisure time PA assessed by questionnaire within 24 months after diagnosis | Median follow-up : 8,1 years |
High recreational PA level (≥8,5 MET-h/week) was associated with lower all-cause mortality. No data on colorectal cancer recurrence |
| Arem and et al. 2015 [70] | Colorectal cancer All cancer stages excepted stage 0 |
Cohort (National Institutes of Health (NIH)–AARP Diet and Health Study) 1759 patients |
Leisure time PA level assessed within 4 years after cancer diagnosis | Median follow-up : 11,3 years |
Postdiagnosis leisure time PA ≥7h/week, compared with none, was associated with a 31% lower all-cause mortality risk. No data on colorectal cancer recurrence. |
| Wang and et al. 2017[85] | Prostate cancer nonmetastatic All cancer stages After diagnosis |
Cohort (Cancer Prevention Study (CPS)-II Nutrition) 5319 men |
Recreational PA questionnaire at least 1 year after diagnosis | Median follow-up: 10 years |
High level of recreational PA (≥17.5 vs 3.5–<8.75 MET-h/week) was associated with a significant 31% lower risk of overall prostate cancer-specific mortality. No data on cancer recurrence. |
| Fridenreich and et al. 2016 [77] | Prostate cancer Stages II-IV After diagnosis |
Cohort (Alberta Cancer Registry) 830 men |
Postdiagnosis PA was measured up to 3 times per patient by questionnaire | Follow-up between 1 and 17 years | High level of PA (>119 vs ≤42 MET-h/week per year) was associated with a significantly lower all-cause mortality risk. No data on cancer recurrence. |
| Bonn and et al. 2015 [72] | Prostate cancer All cancer stages After diagnosis |
Cohort (Swedish National Cancer Register) 4623 men |
Postdiagnosis PA assessed by questionnaire | Follow-up between 1 and 15 years | Statistically significant lower overall mortality rates were found among men engaged in ≥5 recreational MET-h/day, walking/bicycling ≥20 min/day, performing household work ≥1 h/day or exercising ≥1 h/wk, compared with less active men within each activity type. No data on cancer recurrence. |
| Kenfield and et al. 2011 [80] | Prostate cancer nonmetastatic All cancer stages After diagnosis |
Cohort (Health Professionals Follow-Up Study) 2705 patients |
Self-reported leisure time PA assessed every 2 years | Follow-up between 1 and 18 years | Walked ≥90 minutes per week at a normal to very brisk pace had a 46% lower risk of all-cause mortality compared with shorter durations at an easy walking pace. Men with ≥3 hours per week of vigorous activity had a 49% lower risk of all-cause mortality. No data on prostate cancer recurrence. |
| Richman and et al. 2011 [33] | Prostate cancer nonmetastatic All cancer stages After diagnosis |
Cohort (Prostate Strategic Urologic Research Endeavor) 1455 patients |
PA questionnaire every 6 months | Follow-up between 1 and 18 years | Men who walked briskly for 3 h/week or more had a 57% lower rate of cancer recurrence than men who walked at an easy pace for less than 3 h/week. No data on overall survival. |
| Wiskemann and et al. 2015 [29] | Allogeneic stem cell transplant patients | Randomized controlled trial 103 patients |
Duration: 1-4 weeks prior to hospital admission (non-supervised); Supervised sessions during hospitalisation (mean 44 days) ; 8 weeks at home non-supervised Frequency: Combination of endurance (3 to 5 times weekly) and resistance (2 times weekly) exercises |
Follow-up: 2 years after transplatation | Exercise intervention patients trend to lower total mortality rate than controls. The effect on non-relapse mortality was not statiscally significant. |
| Courneya and et al. 2015 [28] | Lymphoma Stages I-IV During treatment |
Randomized controlled trial 122 patients |
Duration: 12 weeks of supervised aerobic exercise
Frequency: 3 times/week Intensity: 15 to 45 minutes on cycloergometer (60-75% VO2peak) PA assessment by questionnaire at 6-month follow-up. |
Median follow-up : 5,1 years | No significant effect of PA program on progression-free survival. Specific trials designed to answer this question are needed. |
| Schmid and et al. 2018 [83] | First primary hematologic cancer | Cohort (Prostate Strategic Urologic Research Endeavor) 5182 patients between 50 and 71 years |
Leisure time PA questionnaire within 1 year after diagnosis | Median follow-up : 4,4 years |
Leisure time PA ≥4 hours/week was significantly associated with all-cause mortality among all hematologic cancer survivors, NHL survivors, myeloma survivors, and leukemia survivor. No data on cancer recurrence. |
| PA physical activity, CWLS Collaborative Women’s Longetivity Study, VO2peak Peak oxygen consumption, MET Metabolic equivalent. | |||||
| Table 2: Characteristics of the 18 randomized controlled trials in progress about the effect of post-diagnosis physical activity intervention on survival and recurrence in cancer patients. | ||||||
| Principal investigator, clinical trial registration number | Cancer type, stage and subjects | PA intervention | PA measurement | Cancer phase of PA intervention | Follow-up duration | Outcome(s) (PE or SE) |
| Nita Nair, DNB MRCS - Mumbai, Maharashtra, India (NCT02161900) |
Breast cancer Stage I-III 850 women |
Duration: No information Frequency: No information Yoga exercises Intensity : No information Supervised : No information |
No information | During treatment | 5 years | Disease-free survival (PE) Overall survival (SE) |
| Livia S Augustin, PhD St. Michael's Hospital (Canada) (NCT02786875) | Breast cancer Stage I-III 506 women |
Duration: 33 months Frequency: Brisk walk of at least 30min/day more than the habitual PA (general recommendation) Intensity: No information Not supervised |
No information | 1 year post-diagnosis | 33 months | Disease-free survival (PE) |
| Inger Thune, MD. PhD Oslo, The Cancer center, Oslo University center, Norway (NCT02240836) | Breast cancer Stage I-II 600 women |
Duration: 12months Frequency: Supervised center training sessions in group 60 minutes twice a week. Informed to exercise at home for at least 120 minutes not supervised Endurance and strenght training Intensity: No information |
No information | During treatment (women newly diagnosed) | 10 years | Relapse of breast cancer disease, Breast Cancer Specific Mortality, Overall mortality, Disease-free survival, Recurrence-free interval (SE) |
| Christina Dieli-Conwright, PhD - University of Southern California/National Cancer Institute (USA) (NCT03091842) |
Breast cancer Stage I-III 300 women with obesity and postmenopausal |
Duration : 16 weeks Frequency: Group 1 : 50 minutes comprising of warm up over 5 minutes, moderate to vigorous aerobic and resistance exercises over 40 minutes, and cool down over 5 minutes 3 days per week – Supervised center sessions Group 2 : 80 minutes comprising of warm up over 5 minutes, aerobic exercise over 15 minutes, resistance exercise over 55 minutes, and cool down over 5 minutes 3 days per week – Supervised center sessions Group 3 : home-based stretching program comprising of one set of 3-4 static stretching exercises held for 30 seconds 3 days per week – Unsupervised sessions Intensity: |
Weekly activity log (only group 3) | During treatment (women newly diagnosed) | 8 years | Disease-free survival overall survival and recurrence free-survival (SE) |
| Pamela J Goodwin, MD, MSc - UHN-Mount Sinai Hospital, Toronto (Canada) (NCT00463489) |
Breast cancer Early stage 2150 postmenopausal women with overweight |
Lifestyle intervention : individual weight loss, diet and physical activity goals, incorporated into a 2-year standardized, structured telephone and mail-based intervention. Recommendation : 150-200 minutes per week of moderate intensity aerobic PA (as walking) |
IPAQ during all evaluation sessions | During treatment (Letrozole) | 8 years | Disease-free survival (PE) Overall survival (SE) |
| Jennifer Ligibel, MD – Dana-Farber Cancer Institute, Boston (USA) (NCT02750826) |
Breast cancer Early stage 3136 women with overweight |
Telephone-based weight loss intervention (2 years) : general recommendations of PA | Self-report of PA during evaluations sessions | 12 months after the first diagnosis | 10 years | Invasive disease-free survival (PE), Overall survival (SE), Distant disease-free survival |
| Antonio AGUDO, MD PhD Institut Catala d’Oncologia – L’Hospitalet, (Spain) (NCT02035631) | Breast cancer Stage I-III 2000 women |
Duration : No information Frequency: Two sessions per week led by trained physical activity monitors (unsupervised) including aerobic exercise of high/moderate intensity, and instruction about the at-home exercise activities (3 more sessions) Intensity : No information |
No information | 3 months post-treatment and 15 months post-diagnosis | 5 years | Time to local and distant recurrence (PE), overall survival (SE), Disease-free survival (SE), |
| Chia-Chin Lin, PhD The University of Hong Kong (China) (NCT03482323) |
Lung cancer Stage IIIb-IV 372 sedentary patients |
Duration : 12 weeks Frequency : 2 sessions/week supervised Group1 : aerobic exercise Group 2 : Tai Chi intervention Intensity : No information |
Accelerometer before/after interventions | During treatment | 1 year | One-year survival rate (PE) |
| Sandy Jack, PhD - University Hospitals Southampton NHS Foundation Trust (England) (NCT03334071) |
Lung cancer Stage IIIb-IV 100 patients |
Duration : 12 weeks Frequency : 2 sessions/week (1 supervised in-hospital and 1 home-based) Intensity : Individually tailored |
Accelerometer before/after interventions |
During treatment | 1 year | Overall survival (SE) |
| Marta Kramer Mikkelsen, MHSc- Rigshospitalet, (Denmark) (NCT03411200) |
Lung cancer locally advanced or metastatic non-small cell lung cancer 100 patients |
Duration : 12 weeks Frequency : 2 sessions/week Supervised and group-based exercise in center two times a week (60 minutes per session). The program consists of warm-up, exercises for balance and flexibility, progressive resistance training, and stretching and relaxation. Intensity: No information |
Accelerometer before/after interventions |
During treatment | 2 years | Overall survival (SE) |
| Joachim Wiskemann, Dr. - National Center for Tumor Diseases, Heideberg (Germany) (NCT02055508) |
Lung cancer Stage IIIb-IV 232 patients |
Duration : 24 weeks Frequency : 3 sessions/week of combined resistance and endurance Program Phase 1 : supervised training sessions Phase 2 : 3x/week at least two/one supervised training sessions Intensity: No information |
No information | During treatment | 1 year | Overall survival, progression-free survival (SE) |
| Viviane Hess, Prof - University Hospital, Basel (Switzerland) (NCT02597075) |
Colorectal cancer Palliative care 524 patients |
Duration : 12 weeks Frequency : 2 sessions/week on cycloergometer in center and a self-paced increase in physical activity during daily life using a pedometer with a daily step goal as a motivational tool. Intensity: No information |
Podometer | During treatment | 1 year | Progression-free survival (PE) Overall survival (SE) |
| Onerup A., PhD - Department of Surgery, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg (Sweden) (NCT02299596) |
Colorectal cancer Planned surgical procedure 620 patients |
Duration : 6 weeks (2 weeks before and 4 weeks after surgical procedure) Frequency : 30 minutes / day of aerobic exercise (chosen by the patient) Intensity: No information |
Questionnaire (IPAQ) | Prior and after surgical procedure | 5 year | Mortality (SE) |
| Katrina A Knight, MBChB - Royal Alexandra Hospita, Paisley (United Kingdom) (NCT03336229) |
Primary operable colorectal cancer 72 patients |
Duration : No information Frequency : No information Graduated walking programme, strengthening exercises and respiratory muscle training (Home-based telephone-guided) Intensity: No information |
No information | Prior surgical procedure | 3 years | Survival (SE) |
| Kerry Courneya, PhD - University of Alberta (Canada) (NCT00819208) |
Colorectal cancer High risk II-III 962 patients PA level < 150 minutes/week |
Duration : 36 months Frequency : Phase 1= Intensive intervention for 6 months Phase 2= Reduced intervention for months 6-12 Phase 3= Minimal intervention for months 12-36 Intensity: Individualized from results to cardiopulmonary exercise test |
Questionnaire and daily record | Post chemotherapy (between 60 to 180 days) | 10 years | Disease-free survival (PE) Overall survival (SE) |
| Robert Newton, Dr - Edith Cowan University Perth (Australia) (NCT02730338) |
Prostate cancer 866 patients |
Duration : 96 weeks Frequency : First 48 weeks = 3 sessions supervised in an exercise clinic setting with gradual transition to self-management (Resistance and aerobic exercises) Subsequent 48 years = self managed exercise with one visit at the beginning of each cycle Intensity: High intensity |
Questionnaire | During treatment | 3 years | Overall survival (PE) Disease progression (SE) |
| Miklos Pless, Prof. - Kantonsspital Winterthur KSW (Switzerland) (NCT02585362) |
Advanced prostate cancer 88 patients |
Duration : 12 weeks Frequency : 2 sessions/week in center and 1 home-based session/week Intensity: No information |
No information | During treatment | 6 months | Overall survival (SE) |
| Jarden M., PhD - Copenhagen University Hospital (Denmark) (NCT01404520) |
70 Patients with leukemia | Duration : 12 weeks Frequency : 3 hour/week supervised in-hospital program of aerobic (stationary cycle) and functional muscle training at high intensity. Combined with relaxation training (low intensity) and unsupervised in-home walking (3 sessions/week) |
Podometer, questionnaire and logbook | During treatment | 1 year | Overall survival (SE) |
| PA physical activity, PE primary endpoint, SE secondary endpoint, IPAQ International physical activity questionnaire. | ||||||
Discussion
An interesting but elusive role of physical activity on cancer survival and recurrence in human patients
By contrast with the well-known messages disseminate in medical literature and public health communication, the systematic review underlines clearly the lack of causal relationship between post diagnosis PA and survival or cancer recurrence.
Of the 31 studies included, only 3 are RCT in the 5 main cancers (Figure 1) [27-29]. The studies incorporating experimental follow-up design reported no significant effect of a PA program on overall mortality, specific mortality or risk of recurrence. As noted by the authors, these studies were not designed or did not have the statistical power (e.g., small number of participants) to show the efficacy of the PA program on these parameters. Nevertheless, observational cohorts indicate positive relationship between PA level and overall survival (23 of 25 cohorts) or specific survival (10 of 14 cohorts) (Table 1). This relationship is described in the broad majority of studies as part of a recreational physical activity practiced daily by patients and not supervised and well-described PA program during and/or after treatments. In addition, based all studies included, 9 reported data on the risk of recurrence in cancer patients. Of these studies, only 4 reported a significant relationship between recreational PA and recurrence risk [30-33].
Figure 1: Number and type of studies published or in progress regarding about the relation between post diagnosis physical activity, survival and recurrence in cancer patient.
All these data issue from literature are supported by 11 meta-analyses [21,23-25,34-40]. It should be noted that all the studies included in meta-analyses are found in our literature review without exception. In these works, the positive relationship between PA level, overall and specific mortality is described only in breast, colon and prostate cancers with increased effects in patients with higher PA levels. For example, the meta-analysis of Schmid et al. [24,] reported a 41% decrease in overall mortality and a 34% decrease in specific mortality in breast cancer patients with physical activity levels ≥ 3MET.h/week. The authors also describe a dose-response effect with decreases of 13% and 34% in overall mortality for increases of 5 MET.h/week and 15 MET.h/week respectively. Similar results have been found in colon and prostate cancers [21,24,35,]. Interestingly, although these meta-analyses did not suggest a causal relationship, the positive effect of PA on overall or specific survival was observed after statistical analyses incorporating adjustment on different clinically relevant variables in cancer (e.g., cancer stage, treatment, smoking status, co-morbidities etc.). Regarding the relationship between the PA level and the risk of recurrence, only meta-analyses performed in breast cancer reported relevant data even if the authors specified that the results must be taken with caution given the small number of studies included in the analysis [21,24,25,34]. Thus, Ibrahim and Al-Homaidh 2011 reported a 24% decreased risk of recurrence in breast cancer patients with AP levels ≥ 3MET.h/week.
However, as mentioned in some meta-analyses and systematic reviews [16,21,41], this narrative review highlights major limitations to be considered in data interpretation from the literature (Table 1).
1/ Change in PA level during post-diagnosis follow-up of patients has rarely been studied in observational cohorts with baseline assessment present in less than 20% of studies [36]. To our knowledge, only one study cohort was interested in the effect of the change in PA level, evaluated 1 year after the baseline assessment, on overall survival but reported no significant relationship [42]. Yet the change in PA level during follow-up is a major consideration in assessing the effect of AP level on survival. For example, it has recently been shown that an increase in PA levels assessed before and after cancer diagnosis was associated with a reduction in long-term mortality [24].
2/ In all cohorts, PA was assessed with declarative methods using long-term recall questionnaires or interviews. This methodology is interesting for epidemiological studies to estimate individual PA dose, but with important risks of bias [43]. Some authors argued that these self-report methods capture only 50% of objectively measured energy expenditure [44]. In this sense, Matthews et al. [43] recommend to combine these questionnaires with objective measure tools such as accelerometer or pedometer. Another alternative should be to repeat frequently self-report during patient follow-up estimating the PA level achieved in short term recall to reduce the risk of bias [43].
3/ The review also points out heterogeneity of cancer and stage follow by observational cohorts. For example, no cohort has been achieved in lung cancer which remains the highest mortality rate cancer (Figure 1). More than 50% of the studies included breast cancer with a large severity, stage I, II or III. These data therefore indicate that it is scientifically inconsistent to overgeneralize the causal relationship between the PA level, overall and specific survival to all cancer types.
4/ The review also underlines the heterogeneity between the beginning and the end (from 0 to 4 years after diagnosis of PA programs, as well as the duration of follow-up (from 1 to 18 years after diagnosis) (Table 1).
5/ Finally, the review indicates substantial differences between PA programs. For example, the cut-offs defined can vary from 3 to 27 MET.h/week depending on the studies and cancer types [45-47]. Most studies use the term of “recreational PA” associated with survival or risk of recurrence. This term includes a broad range of activities such as running, swimming, cycling, gardening, and skiing (etc.) that patients can practice daily without having a rigorous PA program. It is well recognized that an inadequate description of PA in studies is a major factor contributing to the absence of PA prescription by clinicians [48,49].
All these limitations make the analysis and interpretation of these data very complex even after rigorous meta-analyses published. For these reasons, the role of PA and PA supervised programs in survival and recurrence risk remains elusive in patients with cancer as has been suggested by some researchers [16,21,23,41].
Major challenges of research perspectives on PA, survival and recurrence
As it is well recognized in literature, physical inactivity, defined as an PA level of less than 150 minutes per week by several academic organizations [50-52] is a major problem in cancer survivors [14,20]. Although data are heterogeneous across studies, the percentage of inactive patients increased compared to healthy population [51,53,54]. Because of their important side effects, the main antitumor treatments favor this phenomenon in this population [14]. For example, a large French cohort within multiple cancer types showed that about 90% of patients do not increase their level of PA two years after diagnosis and this observation is worsen at 5 years [55]. Thus, the major issue for future research projects is to develop adapted programs to increase the PA level in cancer survivors for regular and self-administered long-term practice, which is essential to improve survival and reduce recurrence risk [56].
The first message widely found in the literature is to carry out rigorous RCT with appropriate designs and well-described PA program. Indeed, it seems essential to evaluate non-pharmacological interventions such as PA programs [48,49]. Overall, the analysis of 137 clinical trials evaluating non-pharmacological interventions showed that 61% did not report sufficient details to be replicated [26,57]. These methodological considerations are necessary to guide physicians and researchers towards the implementation of structured and personalized AP programs, provided that the characteristics of the populations studied are homogenized as much as possible [58].
However, when we read the declaration of RCT in progress on ClinicalTrials.gov, methodological limitations persist. As shown in table 2, only 7 out of the 18 ongoing RCT were specifically designed to investigate the survival or cancer recurrence as primary endpoint. For trials analyzing survival or cancer recurrence as a secondary endpoint, we will therefore find limitations in the interpretation of the data, particularly in relation to the statistical power problem described in numerous RCT already published [27-29]. In this case, it seems important to standardize, at least by cancer type, the parameters evaluated (e.g., duration of follow-up to assess survival) as well as the tools to measure accurately PA in patients as discussed previously [43]. It should also be noted that only one RCT in progress combines two tools to determine PA dose and five use an objective measure such as accelerometer or pedometer (Table 2). This standardization will be easier to compare studies and for meta-analyses [58]. In the same sense, we mentioned the importance of evaluating the change of PA level over the duration of patient follow-up and this has been implemented in four ongoing RCT (Table 2). From a methodological viewpoint, it is also necessary to define precisely the three major components of PA programs, duration, frequency and intensity [14,48,49]. Most of the data available on RCT in progress do not allow to precisely identify the content and intensity of the PA program sessions, although the duration and frequency are generally well described (Table 2).
Beyond these methodological considerations, the content of PA programs should lead to long-term behavior change in cancer survivors. As reported by Hudis and Jones [59], promoting long-term PA is a truly complex task, particularly in cancer. Although the global objective would be for all patients to achieve at least the general recommendations (150 minutes/week of moderate to vigorous PA combined with 2-3 sessions/week of resistance training), it is well recognized that minimal changes in PA levels in inactive patients would produce significant improvements in survival and recurrence risk [14,20]. Yet low adherence to PA interventions associated with inclusion difficulties is frequently reported in clinical trials both during and after the treatment phase [26,60]. Thus, the major currently question is: what tools have been identified in the literature to increase the PA level at long-term in cancer survivors? [26].
From this PA behavior change perspective, taking into account barriers and facilitators to practice is one of the major components [20,61-63]. To date, the main barriers reported in literature differ according to the cancer type, in particular regarding physiological health disorders, even some components seem transverse to all patients: lack of time, geographical location in relation to the hospital center, difficulty of access to practice. However, the broad majority of studies on the PA effects in cancer survivors have been conducted in hospital centers, which may explain the adhesion and inclusion difficulties described [26,60]. As shown in table 2, although many ongoing RCT are being conducted in hospital centers (2/3 of studies), there is growing interest in implementing PA programs integrated into the care pathway based on the facilitators recommended. The two main components to facilitate the practice of long-term regular PA in cancer survivors are home-based interventions and supervised sessions [26,61,64-66]. Indeed, the number of studies evaluating the effects of using new technologies (e.g. telephone, e-mail, web/online support, mobile application etc.) to promote PA among cancer patients continues to increase [64,65]. According to some authors, the evolution of traditional model follow-up in cancer patient centers is necessary and must be reconfigured to correspond to the real needs of patients [65]. To this notion of home-based intervention is added the notion of supervised session. To our knowledge, all the studies carried out or in progress (Table 2) on the implementation and evaluation of home-based programs to induce a PA behavior change in cancer survivors are conducted unsupervised, whether by telephone, e-mail, web support or via mobile applications. The use of these non-face-to-face new technologies, defined as “broad-reaching approaches”, is a financial and feasible interesting solution, but limited and heterogeneous effects on long-term PA levels are reported [64,67]. As reported by Buffart et al. [56], the effects are greater when programs used professional supervision, whether on quality of life, physical ability or patient adherence to the intervention. An important point raised by the authors is that PA sessions should be conducted by a qualified health professional, whether supervised or not [56,65].
A potentially more effective solution evocated in literature to encourage patients towards autonomy in PA practice would be to combine supervised sessions in hospital-center with unsupervised home-based sessions, even if the risks of low adherence to the supervised program remain [26]. Currently, 7 ongoing RCT (Table 2) have used this methodology and the results will allow us to make progress on this point in the coming years. Regarding the intervention durations implemented, whether in published trials or in RCT in progress, we find a significant heterogeneity (from 4 weeks to 2 years) which does not allow us to determine an optimal methodology. Some authors suggest that the 12-month PA program would be too long and would not allow patients to be autonomous in their practice but studies are still needed to show this [26]. In any case, it is important that interventions respect progressiveness in the development of PA interventions. This progressiveness must apply both to the intensity of the sessions but also to the frequency. The implementation of AP programs with a pyramidal design with (1) progressive then degressive evolution of the number of supervised sessions per week associated with (2) an increase of the sessions number in autonomy during follow-up, constitutes an interesting solution to be evaluated scientifically to induce a PA behavior change in cancer survivors. Another innovative solution mentioned in the literature but, to our knowledge, not yet evaluated in cancer patients is the use of videoconferencing, which would make it possible to carry out PA interventions both at home and supervised by a qualified health professional. Another research perspective to be developed would be to integrate PA interventions into comprehensive patient support as recommended in the latest report of the World Cancer Res Fund International. The AP programs would then be combined with adapted nutritional follow-up and education sessions to potentially optimize benefits on survival. For example, disease management program would sensitize the patient to the interest and benefits of PA, on physical abilities, quality of life and survival, which are facilitating components highlighted in the literature [20,62,63]. For all these solutions, future studies on PA integrated in the care pathway should be directed towards a clinical application favoring the implementation of an adapted and personalized medicine directly involving the patient.
From an ethical perspective, many issues need to be addressed in future clinical research. According to some studies, patients would prefer to begin PA interventions after treatment, but this is linked to a lack of patient information and awareness, which further justifies education sessions [20,62]. However, the cancer diagnosis would constitute a “teachable moment” to PA behavior change, even more given the benefits described in the literature regarding PA interventions carried out during treatment [63,68,69]. Another important point is to target the less active patients because of the specific needs of this population and yet it would appear that they are the least involved in clinical trials (inclusion bias) [41]. Finally, the question of setting up a control group is also open to discussion because today it seems difficult to not purpose PA interventions to cancer patients given the current general recommendations. Thus, in future clinical research, it would be appropriate to carry out several groups of interventions by varying different tools and/or modalities: supervised vs unsupervised sessions; place of practice (hospital vs home-based); duration, frequency and intensity of the intervention; individual vs group sessions; follow-up tools (videoconferencing, mobile application, telephone follow-up, etc.).
Conclusion
The PA role on survival and cancer recurrence is based only on observational cohort in the five major solid tumors studied: breast, colorectal, lung, prostate and hematology. The lack of RCT showing causal relationship and the methodological limitations of cohort makes data interpretation complex to generalize. Nevertheless, these observational cohort studies provide many interesting research issues to investigate the specific PA role on survival and recurrence risk. The major challenge of current and future research projects is to develop adapted and personalized PA interventions, tailored as much as possible to the type of cancer, to induce progressively a regular and self-administered long-term practice. For this purpose, it seems essential to develop RCT with rigorous methodologies and adapted PA programs that consider patients “real life” problems. Many innovative solutions exist such as mobile applications, internet support or videoconferencing, but it is now necessary to find PA interventions that induce optimal benefits, using home-based and/or supervised sessions depending on the populations studied, on the PA level, survival and the recurrence risk. These observations and clinical research hypotheses also suggest the potential antitumoral PA role reported in pre-clinical animal studies [17]. Although the underlying mechanisms are still largely unknown in different forms of cancer, some parameters such as inflammation, the immune system or insulin appear to be involved in tumor development. These potentially PA-modulated parameters could also be incorporated into the evaluation of future clinical research projects.
Acknowledgements
The authors gratefully acknowledge the Supportive Care Department team and Research Department team (Institut du Cancer de Montpellier, ICM), the Fondation ARC, the SIRIC Montpellier Cancer (Grant INCa-DGOS-Inserm 6045), and Cedric Baudinet (V@Si company).
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C1, Allen C2, Barber RM2, Barregard L. et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017; 3: 524-528. Ref.: https://goo.gl/qt1GT9
- Lowy DR, Collins FS. Aiming High — Changing the Trajectory for Cancer. N Engl J Med. 2016; 374: 1901–1904. Ref.: https://goo.gl/iom4XT
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018: Cancer Statistics, 2018. CA Cancer J Clin. 2018; 68: 7–30. Ref.: https://goo.gl/ocDvFd
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann Behav Med. 1999; 21: 171–179. Ref.: https://goo.gl/apsLpN
- Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008; CD006145. Ref.: https://goo.gl/DasDop
- Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Clinical Otolaryngology. 2012; CD008465. Ref.: https://goo.gl/DGLyUi
- Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010; 4: 87–100. Ref.: https://goo.gl/KU9xPT
- Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012; 344: e70–e70. Ref.: https://goo.gl/wFfLdU
- Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, et al. Effect of Exercise Training on Peak Oxygen Consumption in Patients with Cancer: A Meta-Analysis. Oncologist. 2011; 16: 112–120. Ref.: https://goo.gl/qHWiPM
- Strasser B, Steindorf K, Wiskemann J, Ulrich CM. Impact of Resistance Training in Cancer Survivors: A Meta-Analysis. Med Sci Sports Exerc. 2013; 45: 2080–2090. Ref.: https://goo.gl/ZdKaSX
- Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, et al. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2012; 104: 815–840. Ref.: https://goo.gl/Y6kkfW
- Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem Soc Trans. 2017; 45: 905–911. Ref.: https://goo.gl/KfXfQT
- Löf M1, Bergström K, Weiderpass E. Physical activity and biomarkers in breast cancer survivors: A systematic review. Maturitas. 2012; 73: 134–142. Ref.: https://goo.gl/BeQVRA
- Ancellin R, Gaillot-de Saintignon J. Bénéfices de l’activité physique pendant et après cancer : des connaissances scientifiques aux repères pratiques. Oncologie. 2017; 19: 95–107. Ref.: https://goo.gl/Njr7mx
- Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, et al. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2016; 25: 1018–1028. Ref.: https://goo.gl/sKFPCE
- Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005-2017). PM R. 2017; 9: S347–S384 Ref.: https://goo.gl/JYc7P3
- Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018; 27: 10–21. Ref.: https://goo.gl/RQcngm
- Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016; 176: 816. Ref.: https://goo.gl/NRjCwU
- Rezende LFM, Sá TH, Markozannes G, Rey-López JP, Lee IM, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2017; 52: 826-833. Ref.: https://goo.gl/xiJCb9
- Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, et al. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology. 2018; 27: 713–724. Ref.: https://goo.gl/248swz
- Benke IN, Leitzmann MF, Behrens G, Schmid D. Physical activity in relation to risk of prostate cancer: a systematic review and meta-analysis. Ann Oncol. 2018; 29: 1154–1179. Ref.: https://goo.gl/Vf37Zn
- Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. The Lancet Oncol. 2017; 18: e457–e471. Ref.: https://goo.gl/KYe7ch
- Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst Rev. 2018; CD011292. Ref.: https://goo.gl/p6tHhv
- Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014; 25: 1293–1311. Ref.: https://goo.gl/MvWPb7
- Wu W, Guo F, Ye J, Li Y, Shi D. Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta-analysis. Oncotarget. 2016; 7: 52095-52103. Ref.: https://goo.gl/VrtrqL
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. Br J Cancer. 2014; 110: 831–841. Ref.: https://goo.gl/pmF7Kt
- Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, et al. Effects of Exercise during Adjuvant Chemotherapy on Breast Cancer Outcomes: Med Sci Sports Exerc. 2014; 46: 1744–1751. Ref.: https://goo.gl/5iSzPu
- Courneya KS, Friedenreich CM, Franco-Villalobos C, Crawford J4, Chua N, et al. Effects of supervised exercise on progression-free survival in lymphoma patients: an exploratory follow-up of the HELP Trial. Cancer Causes & Control. 2015; 26: 269–276. Ref.: https://goo.gl/ipGiWr
- Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, et al. Effects of physical exercise on survival after allogeneic stem cell transplantation: Effects of exercise on survival after transplant. Int J Cancer. 2015; 137: 2749–2756. Ref.: https://goo.gl/2KCGaV
- Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009; 124: 1954–1962. Ref.: https://goo.gl/ABV2hp
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical Activity and Survival after Breast Cancer Diagnosis. JAMA. 2005; 293: 2479-2486. Ref.: https://goo.gl/hNEXdM
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, et al. Physical Activity and Survival after Colorectal Cancer Diagnosis. J Clin Oncol. 2006; 24: 3527–3534. Ref.: https://goo.gl/moCiYp
- Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, et al. Physical Activity after Diagnosis and Risk of Prostate Cancer Progression: Data from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer Res. 2011; 71: 3889–3895. Ref.: https://goo.gl/bRNhgr
- Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011; 28: 753–765. Ref.: https://goo.gl/PYZjca
- Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies: Physical activity and colorectal cancer mortality. Int J Cancer. 2013; 133: 1905–1913. Ref.: https://goo.gl/wV2LLZ
- Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncologica. 2015; 54: 635–654. Ref.: https://goo.gl/FqTDsT
- Lee CHA, Kong JC, Ismail H, Riedel B, Heriot A. Systematic Review and Meta-analysis of Objective Assessment of Physical Fitness in Patients Undergoing Colorectal Cancer Surgery. Dis Colon Rectum. 2018; 1. Ref.: https://goo.gl/Z2uZ5u
- Otto SJ, Korfage IJ, Polinder S, van der Heide A, de Vries E, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2015; 23: 1237–1250. Ref.: https://goo.gl/4S2RhS
- Soares Falcetta F, de Araújo Vianna Träsel H, de Almeida FK, Rangel Ribeiro Falcetta M, Falavigna M, et al. Effects of physical exercise after treatment of early breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2018; 170: 455–476. Ref.: https://goo.gl/VNMdoX
- Zhong S, Jiang T, Ma T, Zhang X, Tang J, et al. Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. Eur J Epidemiol. 2014; 29: 391–404. Ref.: https://goo.gl/zccZaa
- Cormie P, Zopf EM, Zhang X, Schmitz KH. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol Rev. 2017; 39: 71–92. Ref.: https://goo.gl/STh3Vb
- Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011; 22: 427–435. Ref.: https://goo.gl/tA5f4N
- Matthews CE, Moore SC, George SM, Sampson J, Bowles HR. Improving Self-reports of Active and Sedentary Behaviors in Large Epidemiologic Studies. Exerc Sport Sci Rev. 2012; 40: 118-126. Ref.: https://goo.gl/ZHpMWa
- Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy expenditure: how valid are physical activity questionnaires? Am J Clin Nutr. 2008; 87: 279–291. Ref.: https://goo.gl/wkxx7h
- Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012; 131: 637–643. Ref.: https://goo.gl/DQNaqk
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, et al. Physical Activity and Survival after Diagnosis of Invasive Breast Cancer. Cancer Epidemiology Biomarkers & Prevention. 2008; 17: 379–386. Ref.: https://goo.gl/Cx6zyr
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, et al. Impact of Physical Activity on Cancer Recurrence and Survival in Patients With Stage III Colon Cancer: Findings From CALGB 89803. J Clin Oncol. 2006; 24: 3535–3541. Ref.: https://goo.gl/sSGf5J
- Hoffmann TC, Maher CG, Briffa T, Sherrington C, Bennell K, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016; 188: 510–518. Ref.: https://goo.gl/cR2MNz
- Persson G, Brorsson A, Ekvall Hansson E, Troein M, Strandberg EL. Physical activity on prescription (PAP) from the general practitioner’s perspective – a qualitative study. BMC Fam Pract. 2013; 14: Ref.: https://goo.gl/7XXgEf
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, et al. (2012). Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012; 62: 242–274. Ref.: https://goo.gl/WuSLJ7
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med Sci Sports Exerc. 2010; 42: 1409–1426. Ref.: https://goo.gl/Wk8p4S
- Sedentary Behaviour Research Network. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours.” Appl Physiol Nutr Metab. 2012; 37: 540–542. Ref.: https://goo.gl/5RsutP
- Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005; 40: 702–711. Ref.: https://goo.gl/qBH9jG
- Mowls DS, Brame LS, Martinez SA, Beebe LA. Lifestyle behaviors among US cancer survivors. J Cancer Surviv. 2016; 10: 692–698. Ref.: https://goo.gl/rXAN2e
- La vie cinq ans après un diagnostic de cancer. 2018; 364. Ref.: https://goo.gl/p1xTbh
- Buffart LM, Galvão DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: Current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014; 40: 327–340. Ref.: https://goo.gl/1oepk9
- Hoffmann TC, Erueti C, Glasziou PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomized trials. BMJ. 2013; 347: f3755–f3755. Ref.: https://goo.gl/9TYXJK
- Jones LW, Alfano CM. Exercise-oncology research: Past, present, and future. Acta Oncologica. 2013; 52: 195–215. Ref.: https://goo.gl/YKn8Lf
- Hudis CA, Jones L. Promoting exercise after a cancer diagnosis: easier said than done. Br J Cancer. 2014; 110: 829–830. Ref.: https://goo.gl/qqqupY
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, et al. Predictors of Supervised Exercise Adherence during Breast Cancer Chemotherapy. Med Sci Sports Exerc. 2008; 40: 1180–1187. Ref.: https://goo.gl/tVt4aM
- Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015; 149: 331–342. Ref.: https://goo.gl/AkLHxd
- Clifford BK, Mizrahi D, Sandler CX, Barry BK, Simar D, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer. 2018; 26: 685–700. Ref.: https://goo.gl/Z3XDMS
- Demark-Wahnefried W, Rogers LQ, Alfano CM, Thomson CA, Courneya KS, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors: Clinical Interventions for Weight Loss, Diet, and Physical Activity. CA Cancer J Clin. 2015; 65: 167–189. Ref.: https://goo.gl/y6nnwC
- Goode AD, Lawler SP, Brakenridge CL, Reeves MM, Eakin EG. Telephone, print, and Web-based interventions for physical activity, diet, and weight control among cancer survivors: a systematic review. J Cancer Surviv. 2015; 9: 660–682. Ref.: https://goo.gl/F62f7t
- Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013–2018): We still haven’t found what we’re looking for. Cancer Treat Rev. 2018; 69: 188–203. Ref.: https://goo.gl/nRRu78
- Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2018; 15: Ref.: https://goo.gl/N9JLYu
- Eakin EG, Hayes SC, Haas MR, Reeves MM, Vardy JL, et al. Healthy Living after Cancer: a dissemination and implementation study evaluating a telephone-delivered healthy lifestyle program for cancer survivors. BMC Cancer. 2015; 15: Ref.: https://goo.gl/NrHyVZ
- Liss MA, White M, Natarajan L, Parsons JK. Exercise Decreases and Smoking Increases Bladder Cancer Mortality. Clin Genitourin Cancer. 2017; 15: 391–395. Ref.: https://goo.gl/h2XyVr
- Spence RR, Heesch KC, Brown WJ. Colorectal cancer survivors’ exercise experiences and preferences: qualitative findings from an exercise rehabilitation programme immediately after chemotherapy: Exercise rehabilitation study: qualitative findings. Eur J Cancer Care (Engl). 2011; 20: 257–266. Ref.: https://goo.gl/TCfN2o
- Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, et al. Pre- and Postdiagnosis Physical Activity, Television Viewing, and Mortality Among Patients With Colorectal Cancer in the National Institutes of Health–AARP Diet and Health Study. J Clin Oncol. 2015; 33: 180–188. Ref.: https://goo.gl/inrcpK
- Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, et al. The Impact of Body Mass Index and Physical Activity on Mortality among Patients with Colorectal Cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011; 20: 1410–1420. Ref.: https://goo.gl/4Ds5Uh
- Bonn SE, Sjölander A, Lagerros YT, Wiklund F, Stattin P, et al. Physical Activity and Survival among Men Diagnosed with Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2015; 24: 57–64. Ref.: https://goo.gl/EUsoRh
- Bradshaw PT, Ibrahim JG, Khankari N, Cleveland RJ, Abrahamson PE, et al. Post-diagnosis physical activity and survival after breast cancer diagnosis: the Long Island Breast Cancer Study. Breast Cancer Res Treat. 2014; 145: 735–742. Ref.: https://goo.gl/8WobnQ
- Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of Recreational Physical Activity and Leisure Time Spent Sitting with Colorectal Cancer Survival. J Clin Oncol. 2013; 31: 876–885. Ref.: https://goo.gl/uuiKYz
- Chen X, Lu W, Zheng W, Gu K, Matthews CE, et al. Exercise after Diagnosis of Breast Cancer in Association with Survival. Cancer Prev Res (Phila). 2011; 4: 1409–1418. Ref.: https://goo.gl/Rf25Tv
- de Glas NA, Fontein DB, Bastiaannet E, Pijpe A, De Craen AJ, et al. Physical activity and survival of postmenopausal, hormone receptor-positive breast cancer patients: Results of the Tamoxifen Exemestane Adjuvant Multicenter Lifestyle study: Physical Activity in Breast Cancer. Cancer. 2014; 120: 2847–2854. Ref.: https://goo.gl/5ZcUsJ
- Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, et al. Physical Activity and Survival After Prostate Cancer. Eur Urol. 2016; 70: 576–585. Ref.: https://goo.gl/2YE3u8
- Hellmann SS, Thygesen LC, Tolstrup JS, Grønbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010; 19: 366–373. Ref.: https://goo.gl/xXVNqg
- Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B. et al. Physical Activity and Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. Irwin ML1, McTiernan A, Manson JE, Thomson CA, Sternfeld B. 2011; 4: 522–529. Ref.: https://goo.gl/U38mPY
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical Activity and Survival After Prostate Cancer Diagnosis in the Health Professionals Follow-Up Study. J Clin Oncol. 2011; 29: 726–732. Ref.: https://goo.gl/r326eN
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT. et al. Physical Activity and Male Colorectal Cancer Survival. Arch Intern Med. 2011; 169: 2102. Ref.: https://goo.gl/jd9tba
- Nechuta S, Chen WY, Cai H, Poole EM, Kwan ML, et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 2016; 138: 2088–2097. https://goo.gl/yXc1QL
- Schmid D, Behrens G, Arem H, Hart C, Herr W, et al. Pre- and post-diagnosis physical activity, television viewing, and mortality among hematologic cancer survivors. PLOS ONE. 2018; 13: e0192078. Ref.: https://goo.gl/PCD8uC
- Sternfeld B, Weltzien E, Quesenberry CP Jr, Castillo AL, Kwan M, et al. Physical Activity and Risk of Recurrence and Mortality in Breast Cancer Survivors: Findings from the LACE Study. Cancer Epidemiol Biomarkers Prev. 2009; 18: 87–95. Ref.: https://goo.gl/4eVv2B
- Wang Y, Jacobs EJ, Gapstur SM, Maliniak ML, Gansler T, et al. Recreational Physical Activity in Relation to Prostate Cancer–specific Mortality Among Men with Nonmetastatic Prostate Cancer. Eur Urol. 2017; 72: 931–939. Ref.: https://goo.gl/nL1kD7
- Williams PT. Significantly greater reduction in breast cancer mortality from post-diagnosis running than walking: Post-diagnosis exercise vs breast cancer mortality. Int J Cancer. 2014; 135: 1195–1202. Ref.: https://goo.gl/zRmgNj